Treatment of Alzheimer's Disease with Repetitive Transcranial Magnetic Stimulation Combined with Cognitive Training: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study
- PMID: 26365021
- PMCID: PMC4712287
- DOI: 10.3988/jcn.2016.12.1.57
Treatment of Alzheimer's Disease with Repetitive Transcranial Magnetic Stimulation Combined with Cognitive Training: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study
Abstract
Background and purpose: Repetitive transcranial magnetic stimulation (rTMS) has been examined as a potential treatment for many neurological disorders. High-frequency rTMS in particular improves cognitive functions such as verbal fluency and memory. This study explored the effect of rTMS combined with cognitive training (rTMS-COG) on patients with Alzheimer's disease (AD).
Methods: A prospective, randomized, double-blind, placebo-controlled study was performed with 27 AD patients (18 and 8 in the treatment and sham groups, respectively, and 1 drop-out). The participants were categorized into mild [Mini-Mental State Examination (MMSE) score=21-26] and moderate (MMSE score=18-20) AD groups. The rTMS protocols were configured for six cortical areas (both dorsolateral prefrontal and parietal somatosensory associated cortices and Broca's and Wernicke's areas; 10 Hz, 90-110% intensity, and 5 days/week for 6 weeks). Neuropsychological assessments were performed using the AD Assessment Scale-cognitive subscale (ADAS-cog), Clinical Global Impression of Change (CGIC), and MMSE before, immediately after, and 6 weeks after the end of rTMS-COG treatment.
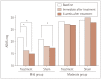
Results: Data from 26 AD patients were analyzed in this study. There was no significant interactive effect of time between the groups. The ADAS-cog score in the treatment group was significantly improved compared to the sham group (4.28 and 5.39 in the treatment group vs. 1.75 and 2.88 in the sham group at immediately and 6 weeks after treatment, respectively). The MMSE and CGIC scores were also improved in the treatment group. Based on subgroup analysis, the effect of rTMS-COG was superior for the mild group compared to the total patients, especially in the domains of memory and language.
Conclusions: The present results suggest that rTMS-COG represents a useful adjuvant therapy with cholinesterase inhibitors, particularly during the mild stage of AD. The effect of rTMS-COG was remarkable in the memory and language domains, which are severely affected by AD.
Keywords: Alzheimer's disease; cognitive therapy; repetitive transcranial magnetic stimulation.
Conflict of interest statement
Figures


References
-
- Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer's disease. Arch Med Res. 2012;43:600–608. - PubMed
-
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

