Metalloprotease OMA1 Fine-tunes Mitochondrial Bioenergetic Function and Respiratory Supercomplex Stability
- PMID: 26365306
- PMCID: PMC4568518
- DOI: 10.1038/srep13989
Metalloprotease OMA1 Fine-tunes Mitochondrial Bioenergetic Function and Respiratory Supercomplex Stability
Abstract
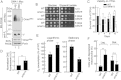
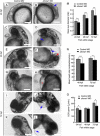
Mitochondria are involved in key cellular functions including energy production, metabolic homeostasis, and apoptosis. Normal mitochondrial function is preserved by several interrelated mechanisms. One mechanism - intramitochondrial quality control (IMQC) - is represented by conserved proteases distributed across mitochondrial compartments. Many aspects and physiological roles of IMQC components remain unclear. Here, we show that the IMQC protease Oma1 is required for the stability of the respiratory supercomplexes and thus balanced and tunable bioenergetic function. Loss of Oma1 activity leads to a specific destabilization of respiratory supercomplexes and consequently to unbalanced respiration and progressive respiratory decline in yeast. Similarly, experiments in cultured Oma1-deficient mouse embryonic fibroblasts link together impeded supercomplex stability and inability to maintain proper respiration under conditions that require maximal bioenergetic output. Finally, transient knockdown of OMA1 in zebrafish leads to impeded bioenergetics and morphological defects of the heart and eyes. Together, our biochemical and genetic studies in yeast, zebrafish and mammalian cells identify a novel and conserved physiological role for Oma1 protease in fine-tuning of respiratory function. We suggest that this unexpected physiological role is important for cellular bioenergetic plasticity and may contribute to Oma1-associated disease phenotypes in humans.
Figures





References
-
- Vafai S. B. & Mootha V. K. Mitochondrial disorders as windows into an ancient organelle. Nature 491, 374–383 (2012). - PubMed
-
- Figueira T. R. et al. Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid. Redox Signal. 18, 2029–2074 (2013). - PubMed
-
- Baker B. M. & Haynes C. M. Mitochondrial protein quality control during biogenesis and aging. Trends Biochem. Sci. 36, 254–261 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

