Putting on the brakes: Bacterial impediment of wound healing
- PMID: 26365869
- PMCID: PMC4650533
- DOI: 10.1038/srep14003
Putting on the brakes: Bacterial impediment of wound healing
Abstract
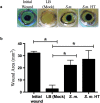
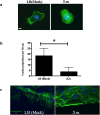
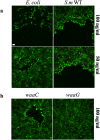
The epithelium provides a crucial barrier to infection, and its integrity requires efficient wound healing. Bacterial cells and secretomes from a subset of tested species of bacteria inhibited human and porcine corneal epithelial cell migration in vitro and ex vivo. Secretomes from 95% of Serratia marcescens, 71% of Pseudomonas aeruginosa, 29% of Staphylococcus aureus strains, and other bacterial species inhibited epithelial cell migration. Migration of human foreskin fibroblasts was also inhibited by S. marcescens secretomes indicating that the effect is not cornea specific. Transposon mutagenesis implicated lipopolysaccharide (LPS) core biosynthetic genes as being required to inhibit corneal epithelial cell migration. LPS depletion of S. marcescens secretomes with polymyxin B agarose rendered secretomes unable to inhibit epithelial cell migration. Purified LPS from S. marcescens, but not from Escherichia coli or S. marcescens strains with mutations in the waaG and waaC genes, inhibited epithelial cell migration in vitro and wound healing ex vivo. Together these data suggest that S. marcescens LPS is sufficient for inhibition of epithelial wound healing. This study presents a novel host-pathogen interaction with implications for infections where bacteria impact wound healing and provides evidence that secreted LPS is a key factor in the inhibitory mechanism.
Figures




References
-
- Wilson S. E. et al. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res 20, 625–637 (2001). - PubMed
-
- Reim M., Kottek A. & Schrage N. The Cornea Surface and Wound Healing. Prog Retin Eye Res 16, 183–225 (1997).
-
- Spurr-Michaud S. J., Barza M. & Gipson I. K. An organ culture system for study of adherence of Pseudomonas aeruginosa to normal and wounded corneas. Investigative Ophthalmology & Visual Science 29, 379–386 (1988). - PubMed
-
- Klotz S. A., Au Y. K. & Misra R. P. A partial-thickness epithelial defect increases the adherence of Pseudomonas aeruginosa to the cornea. Investigative Ophthalmology & Visual Science 30, 1069–1074 (1989). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

