Efficacy and safety of once-daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomized, placebo-controlled study
- PMID: 26366068
- PMCID: PMC4562726
- DOI: 10.2147/COPD.S81053
Efficacy and safety of once-daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomized, placebo-controlled study
Abstract
Background: Combination of the inhaled long-acting muscarinic antagonist umeclidinium (UMEC; GSK573719) with the long-acting β2-agonist vilanterol (VI) is an approved maintenance treatment for COPD in the US and EU. We compared the efficacy and safety of UMEC/VI with placebo in patients with COPD of Asian ancestry.
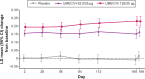
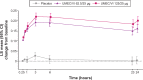
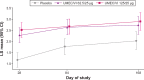
Patients and methods: In this 24-week, Phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study, patients were randomized 1:1:1 to UMEC/VI 125/25 μg, UMEC/VI 62.5/25 μg, or placebo. The primary efficacy end point was trough forced expiratory volume in 1 second (FEV1) on day 169; secondary end points were Transition Dyspnea Index (TDI) focal score at week 24 and weighted mean (WM) FEV1 over 0-6 hours postdose on day 1. Additional end points and safety were also assessed.
Results: Both UMEC/VI 125/25 μg and UMEC/VI 62.5/25 μg statistically significantly improved trough FEV1 at day 169 versus placebo (UMEC/VI 125/25 μg, 0.216 L, [95% confidence interval [CI] 0.175-0.257]; UMEC/VI 62.5/25 μg, 0.151 L, 95% CI 0.110-0.191; both P<0.001). Statistically significant improvements in TDI score were observed for both UMEC/VI groups versus placebo (UMEC/VI 125/25 μg, 0.9, 95% CI 0.3-1.4, P=0.002; UMEC/VI 62.5/25 μg, 0.7, 95% CI 0.1-1.2, P=0.016). On day 1, both UMEC/VI groups improved 0-6-hour WM FEV1 versus placebo (UMEC/VI 125/25 μg, 0.182 L 95% CI 0.161-0.203; UMEC/VI 62.5/25 μg, 0.160 L, 95% CI 0.139-0.181; both P<0.001). Statistically significant improvements for UMEC/VI groups versus placebo were observed for rescue albuterol use at weeks 1-24 (puffs/day, both P<0.001). The incidence of adverse events was similar across groups.
Conclusion: In Asian patients with COPD, once-daily UMEC/VI 125/25 μg and UMEC 62.5/25 μg resulted in clinically meaningful and statistically significant improvements in lung-function end points versus placebo. Symptomatic and quality of life measures also improved. The safety profile of UMEC/VI was consistent with previous studies.
Keywords: Asian; chronic obstructive pulmonary disease; umeclidinium; vilanterol.
Figures

References
-
- Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. - PubMed
-
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. - PubMed
-
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. - PubMed
-
- Jones PW. St George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

