Nanoemulsions containing a synthetic chalcone as an alternative for treating cutaneous leshmaniasis: optimization using a full factorial design
- PMID: 26366075
- PMCID: PMC4562752
- DOI: 10.2147/IJN.S83929
Nanoemulsions containing a synthetic chalcone as an alternative for treating cutaneous leshmaniasis: optimization using a full factorial design
Abstract
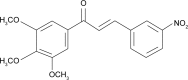
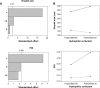
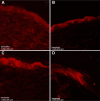
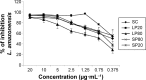
Nanoemulsions are drug delivery systems that may increase the penetration of lipophilic compounds through the skin, enhancing their topical effect. Chalcones are compounds of low water solubility that have been described as promising molecules for the treatment of cutaneous leishmaniasis (CL). In this context, the aim of this work was to optimize the development of a nanoemulsion containing a synthetic chalcone for CL treatment using a 2(2) full factorial design. The formulations were prepared by spontaneous emulsification and the experimental design studied the influence of two independent variables (type of surfactant - soybean lecithin or sorbitan monooleate and type of co-surfactants - polysorbate 20 or polysorbate 80) on the physicochemical characteristics of the nanoemulsions, as well as on the skin permeation/retention of the synthetic chalcone in porcine skin. In order to evaluate the stability of the systems, the antileishmanial assay was performed against Leishmania amazonensis 24 hours and 60 days after the preparation of the nanoemulsions. The formulation composed of soybean lecithin and polysorbate 20 presented suitable physicochemical characteristics (droplet size 171.9 nm; polydispersity index 0.14; zeta potential -39.43 mV; pH 5.16; and viscosity 2.00 cP), drug content (91.09%) and the highest retention in dermis (3.03 µg·g(-1)) - the main response of interest - confirmed by confocal microscopy. This formulation also presented better stability of leishmanicidal activity in vitro against L. amazonensis amastigote forms (half maximal inhibitory concentration value 0.32±0.05 µM), which confirmed the potential of the nanoemulsion soybean lecithin and polysorbate 20 for CL treatment.
Keywords: chalcone; full factorial; leishmaniasis; nanoemulsion; skin permeation.
Figures





References
-
- Myler PJ, Fasel N. Leishmania After the Genome. Seattle: Caister Academic Press; 2008.
-
- World Health Organization . Control of Leishmaniases. Report. Geneva: World Health Organization; 2010. (WHO Technical Report Series, 949). - PubMed
-
- Ben Salah A, Zakraoui H, Zaatour A, et al. A randomized, placebo-controlled trial in Tunisia treating cutaneous leishmaniasis with paromomycin ointment. Am J Trop Med Hyg. 1995;53(2):162–166. - PubMed
-
- Ben Salah A, Ben Messaoud N, Guedri E, et al. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N Engl J Med. 2013;368(6):524–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

