Cross talk between poly(ADP-ribose) polymerase 1 methylation and oxidative stress involved in the toxic effect of anatase titanium dioxide nanoparticles
- PMID: 26366077
- PMCID: PMC4562766
- DOI: 10.2147/IJN.S88059
Cross talk between poly(ADP-ribose) polymerase 1 methylation and oxidative stress involved in the toxic effect of anatase titanium dioxide nanoparticles
Abstract
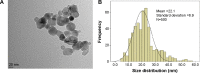
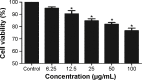
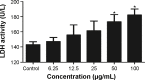
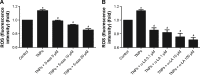
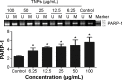
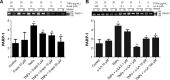
Given the tremendous growth in the application of titanium dioxide nanoparticles (TNPs), concerns about the potential health hazards of TNPs to humans have been raised. Poly(ADP-ribose) polymerase 1 (PARP-1), a highly conserved DNA-binding protein, is involved in many molecular and cellular processes. Limited data demonstrated that certain nanomaterials induced the aberrant hypermethylation of PARP-1. However, the mechanism involved in TNP-induced PARP-1 abnormal methylation has not been studied. A549 cells were incubated with anatase TNPs (22.1 nm) for 24 hours pretreatment with or without methyltransferase inhibitor 5-aza-2'-deoxycytidine and the reactive oxygen species (ROS) scavenger α-lipoic acid to assess the possible role of methylation and ROS in the toxic effect of TNPs. After TNPs characterization, a battery of assays was performed to evaluate the toxic effect of TNPs, PARP-1 methylation status, and oxidative damage. Results showed that TNPs decreased the cell viability in a dose-dependent manner, in accordance with the increase of lactate dehydrogenase activity, which indicated membrane damage of cells. Similar to the high level of PARP-1 methylation, the generation of ROS was significantly increased after exposure to TNPs for 24 hours. Furthermore, α-lipoic acid decreased TNP-induced ROS generation and then attenuated TNP-triggered PARP-1 hypermethylation. Meanwhile, 5-aza-2'-deoxycytidine simultaneously decreased the ROS generation induced by TNPs, resulting in the decline of PARP-1 methylation. In summary, TNPs triggered the aberrant hypermethylation of the PARP-1 promoter and there was a cross talk between oxidative stress and PARP-1 methylation in the toxic effect of TNPs.
Keywords: DNA methylation; PARP-1; oxidative stress; titanium dioxide nanoparticles.
Figures

References
-
- Wang J, Zhou G, Chen C, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168(2):176–185. - PubMed
-
- Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology. 2007;230(1):90–104. - PubMed
-
- Bermudez E, Mangum JB, Wong BA, et al. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004;77(2):347–357. - PubMed
-
- Baggs RB, Ferin J, Oberdorster G. Regression of pulmonary lesions produced by inhaled titanium dioxide in rats. Vet Pathol. 1997;34(6):592–597. - PubMed
-
- Wu J, Sun J, Xue Y. Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicol Lett. 2010;199(3):269–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

