Induction of active demethylation and 5hmC formation by 5-azacytidine is TET2 dependent and suggests new treatment strategies against hepatocellular carcinoma
- PMID: 26366235
- PMCID: PMC4567821
- DOI: 10.1186/s13148-015-0133-x
Induction of active demethylation and 5hmC formation by 5-azacytidine is TET2 dependent and suggests new treatment strategies against hepatocellular carcinoma
Abstract
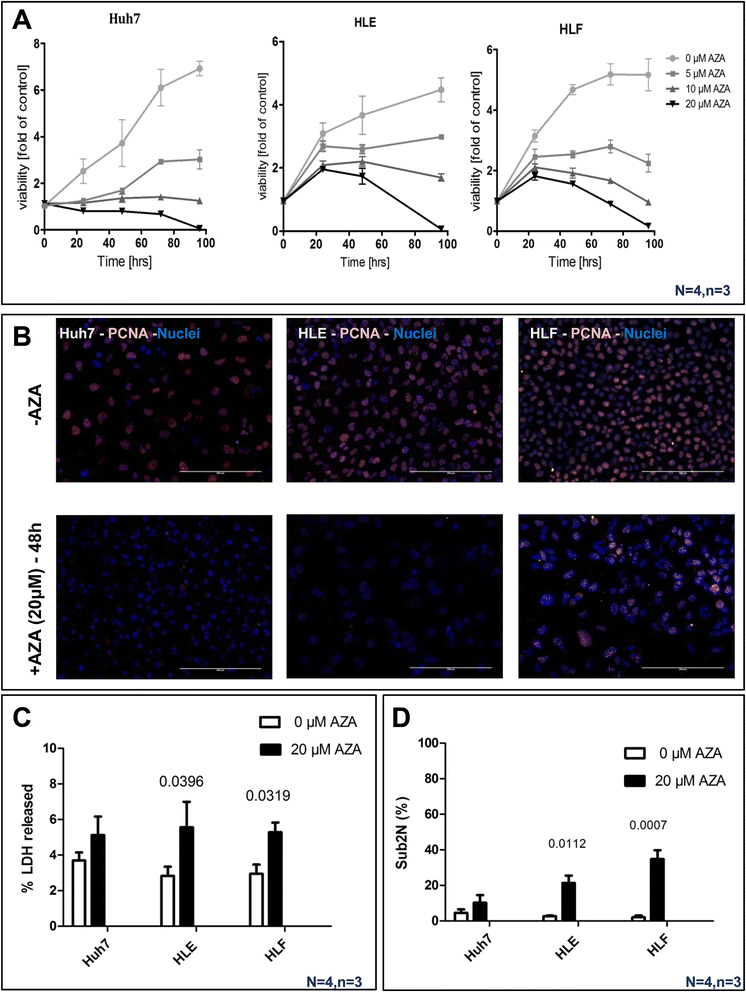
Background: Global deregulation of DNA methylation is one of the crucial causes of hepato cellular carcinoma (HCC). It has been reported that the anti-cancer drug 5-azacytidine (5-AZA) mediates the activation of tumor suppressor genes through passive demethylation by inhibiting DNMT1. Recent evidence suggests that active demethylation which is mediated by ten-eleven translocation (TET) proteins may also be an important step to control global methylation. However, there exists a controversial discussion in which TET proteins are involved in the demethylation process in HCC. Therefore, we firstly wanted to identify which of the TETs are involved in demethylation and later to study whether or not 5-AZA could trigger the TET-dependent active demethylation process in HCC. HCC cell lines (Huh-7, HLE, HLF), primary human hepatocytes (hHeps), and tissues from both healthy (55 patients) and HCC patients (55 patients) were included in this study; mRNA levels of isocitrate dehydrogenase (IDH1, 2) and TETs (TET1-3) were studied via qPCR and confirmed by Western blot. The expression of 5hmC/5mC was determined by immunohistochemistry in human HCC tissues and the corresponding adjacent healthy liver. HCC cell lines were stimulated with 5-AZA (0-20 μM) and viability (Resazurin conversion), toxicity (LDH release), proliferation (PCNA), and 5hmC/5mC distribution were assessed. In addition, knockdown experiments on TET proteins in HCC cell lines using short interference RNAs (siRNAs), in the presence and absence of 5-AZA, were performed.
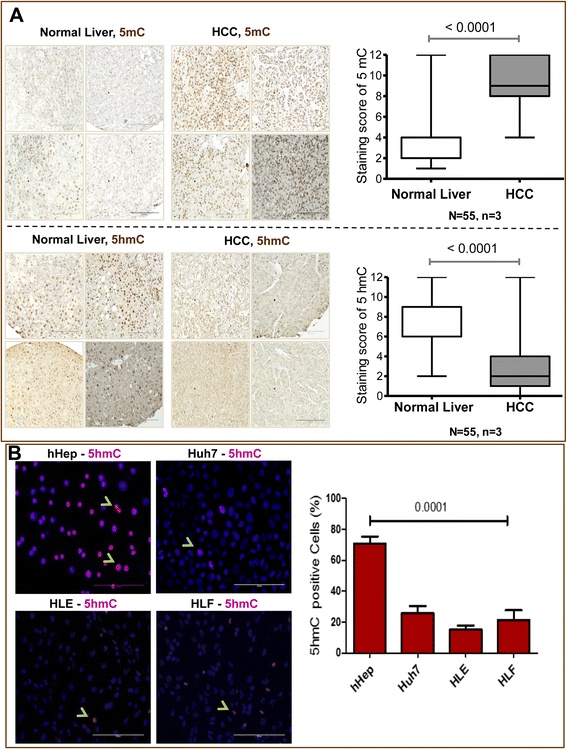
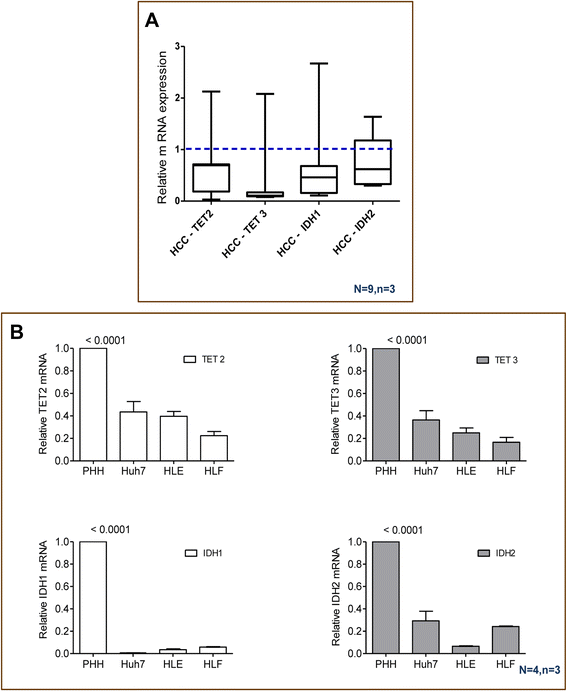
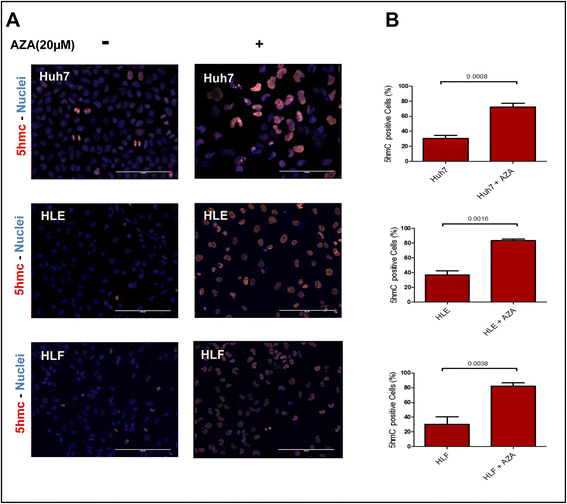
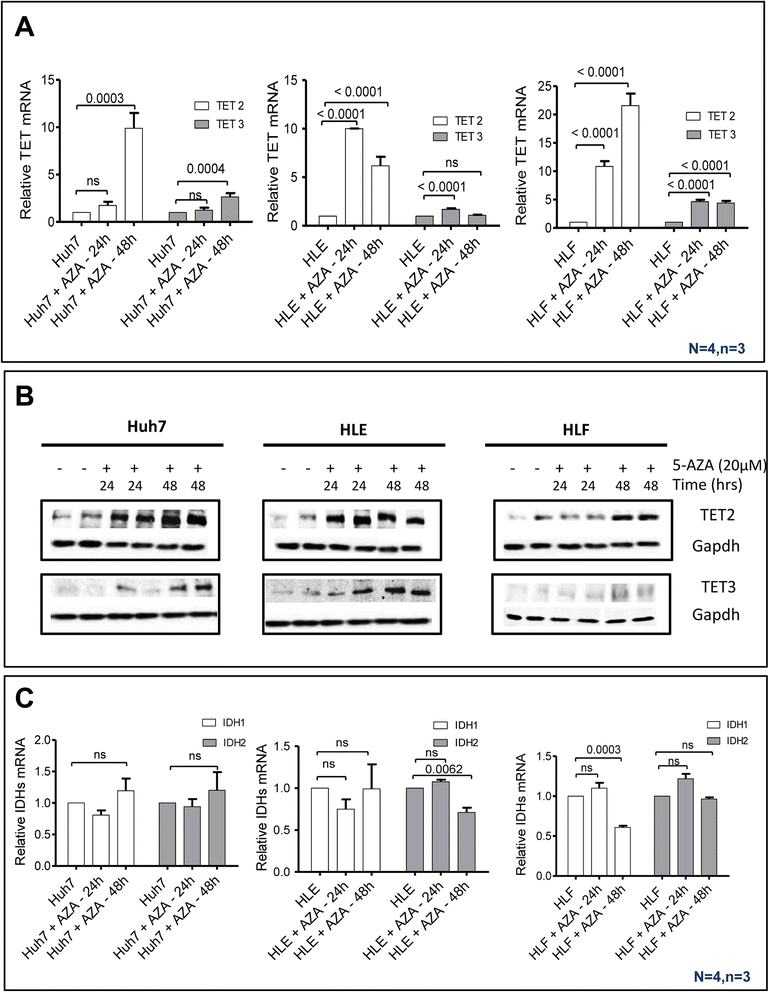
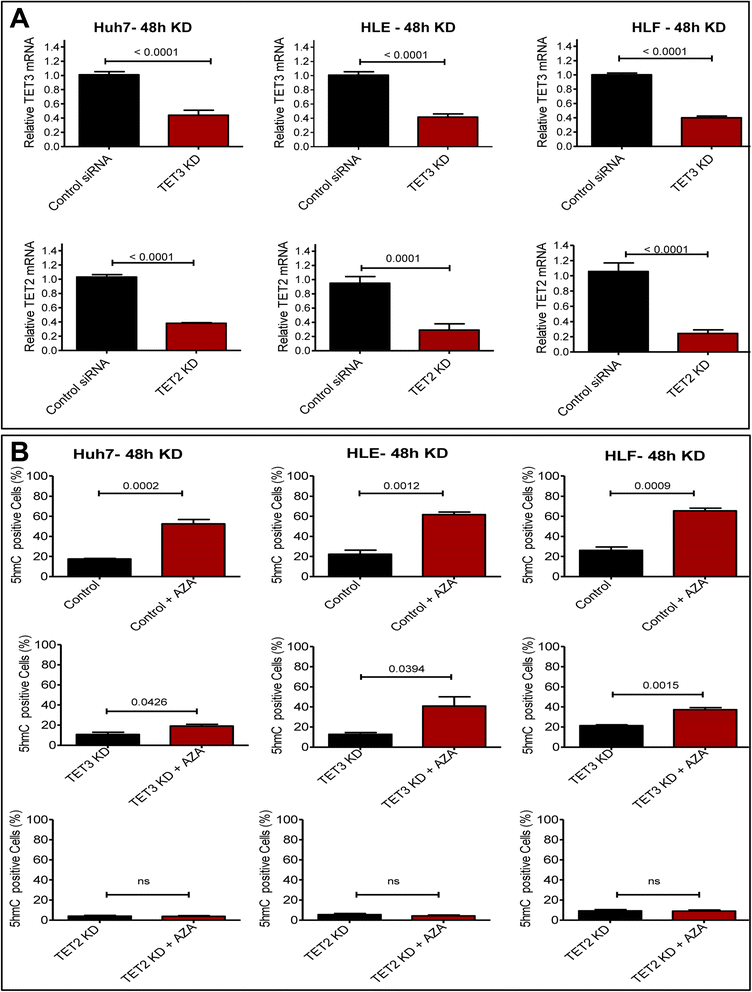
Results: Our data applying qPCR, immunofluorescence, and Western blotting clearly show that TET2 and TET3 but not TET1 were significantly decreased in HCC tissue and different HCC cell lines compared to non-tumor liver tissues and hHeps. In addition, we show here for the first time applying knockdown experiments that 5-AZA is able to trigger an active TET2-dependent demethylation process with concomitant significant changes in 5hmC/5mC in HCC cell lines and hHeps.
Conclusions: Our data clearly show that the expression and activity of TET2 and TET3 proteins but not TET1 are impaired in hepatocellular carcinoma leading to the reduction of 5hmC in HCCs. Furthermore, this study identified a novel function of 5-azacytidine in promoting a TET-mediated generation of 5hmC suggesting that the availability of 5-AZA in cancer cells will have various effects on different epigenetic targets. These findings may open new therapeutic strategies for epigenetic drugs to treat HCC.
Keywords: 5-Azacytidine; 5-Hydroxymethylcytosine; Cancer biomarker; DNA methylation; TET.
Figures
Similar articles
-
Vitamin C enhances epigenetic modifications induced by 5-azacytidine and cell cycle arrest in the hepatocellular carcinoma cell lines HLE and Huh7.Clin Epigenetics. 2016 Apr 30;8:46. doi: 10.1186/s13148-016-0213-6. eCollection 2016. Clin Epigenetics. 2016. PMID: 27134688 Free PMC article.
-
5-Hydroxymethylcytosine-mediated active demethylation is required for mammalian neuronal differentiation and function.Elife. 2021 Dec 17;10:e66973. doi: 10.7554/eLife.66973. Elife. 2021. PMID: 34919053 Free PMC article.
-
Distinct and overlapping control of 5-methylcytosine and 5-hydroxymethylcytosine by the TET proteins in human cancer cells.Genome Biol. 2014 Jun 23;15(6):R81. doi: 10.1186/gb-2014-15-6-r81. Genome Biol. 2014. PMID: 24958354 Free PMC article.
-
Role of ten-eleven translocation proteins and 5-hydroxymethylcytosine in hepatocellular carcinoma.Cell Prolif. 2019 Jul;52(4):e12626. doi: 10.1111/cpr.12626. Epub 2019 Apr 29. Cell Prolif. 2019. PMID: 31033072 Free PMC article. Review.
-
TET enzymes and 5hmC epigenetic mark: new key players in carcinogenesis and progression in gynecological cancers.Eur Rev Med Pharmacol Sci. 2024 Feb;28(3):1123-1134. doi: 10.26355/eurrev_202402_35349. Eur Rev Med Pharmacol Sci. 2024. PMID: 38375718 Review.
Cited by
-
Pan-cancer analyses reveal the molecular and clinical characteristics of TET family members and suggests that TET3 maybe a potential therapeutic target.Front Pharmacol. 2024 Jul 22;15:1418456. doi: 10.3389/fphar.2024.1418456. eCollection 2024. Front Pharmacol. 2024. PMID: 39104395 Free PMC article.
-
Colorectal cancer progression to metastasis is associated with dynamic genome-wide biphasic 5-hydroxymethylcytosine accumulation.BMC Biol. 2025 Apr 16;23(1):100. doi: 10.1186/s12915-025-02205-y. BMC Biol. 2025. PMID: 40241172 Free PMC article.
-
Ten-Eleven Translocation Family Proteins: Structure, Biological Functions, Diseases, and Targeted Therapy.MedComm (2020). 2025 Jul 1;6(7):e70245. doi: 10.1002/mco2.70245. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40599235 Free PMC article. Review.
-
Antileishmanial potentials of azacitidine and along with meglumine antimoniate on Leishmania major: In silico prediction and in vitro analysis.PLoS One. 2023 Sep 8;18(9):e0291321. doi: 10.1371/journal.pone.0291321. eCollection 2023. PLoS One. 2023. PMID: 37682979 Free PMC article.
-
Epigenetic Deregulation of the Histone Methyltransferase KMT5B Contributes to Malignant Transformation in Glioblastoma.Front Cell Dev Biol. 2021 Aug 10;9:671838. doi: 10.3389/fcell.2021.671838. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34447744 Free PMC article.
References
-
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

