The expanding world of hybrid perovskites: materials properties and emerging applications
- PMID: 26366326
- PMCID: PMC4563667
- DOI: 10.1557/mrc.2015.6
The expanding world of hybrid perovskites: materials properties and emerging applications
Abstract
Figures




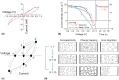
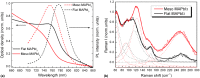


References
-
- Green M.A., Emery K., Hishikawa Y., Warta W., and Dunlop E.D.: Solar cell efficiency tables (version 45). Prog. Photovolt. Res. Appl. 23, 1 (2015).
-
- Kojima A., Teshima K., Shirai Y., and Miyasaka T.: Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050 (2009). - PubMed
-
- Kim H.-S., Lee C.-R., Im J.-H., Lee K.-B., Moehl T., Marchioro A., Moon S.-J., Humphry-Baker R., Yum J.-H., Moser J.E., Grätzel M., and Park N.-G.: Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2, 591 (2012). - PMC - PubMed
-
- Liu M., Johnston M.B., and Snaith H.J.: Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501, 395 (2013). - PubMed
-
- Luo J., Im J.-H., Mayer M.T., Schreier M., Nazeeruddin M.K., Park N.-G., Tilley S.D., Fan H.J., and Gratzel M.: Water photolysis at 12.3% efficiency via perovskite photovoltaics and earth-abundant catalysts. Science 345, 1593 (2014). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
