Mammalian pre-implantation chromosomal instability: species comparison, evolutionary considerations, and pathological correlations
- PMID: 26366555
- PMCID: PMC7857118
- DOI: 10.3109/19396368.2015.1073406
Mammalian pre-implantation chromosomal instability: species comparison, evolutionary considerations, and pathological correlations
Abstract
Pre-implantation embryo development in mammals begins at fertilization with the migration and fusion of the maternal and paternal pro-nuclei, followed by the degradation of inherited factors involved in germ cell specification and the activation of embryonic genes required for subsequent cell divisions, compaction, and blastulation. The majority of studies on early embryogenesis have been conducted in the mouse or non-mammalian species, often requiring extrapolation of the findings to human development. Given both conserved similarities and species-specific differences, however, even comparison between closely related mammalian species may be challenging as certain aspects, including susceptibility to chromosomal aberrations, varies considerably across mammals. Moreover, most human embryo studies are limited to patient samples obtained from in vitro fertilization (IVF) clinics and donated for research, which are generally of poorer quality and produced with germ cells that may be sub-optimal. Recent technical advances in genetic, epigenetic, chromosomal, and time-lapse imaging analyses of high quality whole human embryos have greatly improved our understanding of early human embryogenesis, particularly at the single embryo and cell level. This review summarizes the major characteristics of mammalian pre-implantation development from a chromosomal perspective, in addition to discussing the technological achievements that have recently been developed to obtain this data. We also discuss potential translation to clinical applications in reproductive medicine and conclude by examining the broader implications of these findings for the evolution of mammalian species and cancer pathology in somatic cells.
Keywords: Aneuploidy; IVF; cancer; chromothripsis; embryo; evolution; fragmentation; micronuclei; preimplantation.
Conflict of interest statement
Declaration of interest
The authors report no conflicts of interest.
Figures


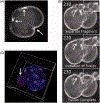
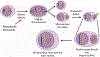
Similar articles
-
Chromosomal instability in mammalian pre-implantation embryos: potential causes, detection methods, and clinical consequences.Cell Tissue Res. 2016 Jan;363(1):201-225. doi: 10.1007/s00441-015-2305-6. Epub 2015 Nov 21. Cell Tissue Res. 2016. PMID: 26590822 Free PMC article. Review.
-
Time-Lapse Imaging for the Detection of Chromosomal Abnormalities in Primate Preimplantation Embryos.Methods Mol Biol. 2018;1769:293-317. doi: 10.1007/978-1-4939-7780-2_19. Methods Mol Biol. 2018. PMID: 29564832
-
Extended in vitro culture of human embryos demonstrates the complex nature of diagnosing chromosomal mosaicism from a single trophectoderm biopsy.Hum Reprod. 2019 Apr 1;34(4):758-769. doi: 10.1093/humrep/dez012. Hum Reprod. 2019. PMID: 30838420
-
Aneuploidy and early human embryo development.Hum Mol Genet. 2008 Apr 15;17(R1):R10-5. doi: 10.1093/hmg/ddn170. Hum Mol Genet. 2008. PMID: 18632690 Review.
-
Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study.Hum Reprod. 2016 Oct;31(10):2245-54. doi: 10.1093/humrep/dew183. Epub 2016 Sep 2. Hum Reprod. 2016. PMID: 27591227
Cited by
-
Chromosome Division in Early Embryos-Is Everything under Control? And Is the Cell Size Important?Int J Mol Sci. 2024 Feb 9;25(4):2101. doi: 10.3390/ijms25042101. Int J Mol Sci. 2024. PMID: 38396778 Free PMC article. Review.
-
Mosaicism in Preimplantation Human Embryos: When Chromosomal Abnormalities Are the Norm.Trends Genet. 2017 Jul;33(7):448-463. doi: 10.1016/j.tig.2017.04.001. Epub 2017 Apr 28. Trends Genet. 2017. PMID: 28457629 Free PMC article. Review.
-
Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential.Nat Commun. 2016 Mar 29;7:11165. doi: 10.1038/ncomms11165. Nat Commun. 2016. PMID: 27021558 Free PMC article.
-
Prospects of Germline Nuclear Transfer in Women With Diminished Ovarian Reserve.Front Endocrinol (Lausanne). 2021 Feb 22;12:635370. doi: 10.3389/fendo.2021.635370. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33692760 Free PMC article. Review.
-
A Novel De Novo Chromosomal Insertion, 46 XX, ins(7:13)(p14; q14.2q21.1) is Related to the Embryo Development Arrest Following Assisted Reproductive Technique.J Reprod Infertil. 2020 Oct-Dec;21(4):308-311. doi: 10.18502/jri.v21i4.4325. J Reprod Infertil. 2020. PMID: 33209748 Free PMC article.
References
-
- Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C and Scott RT (1999) Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril 71:836–842. - PubMed
-
- Alizadeh Z, Kageyama S and Aoki F (2005) Degradation of maternal mRNA in mouse embryos: Selective degradation of specific mRNAs after fertilization. Mol Reprod Dev 72:281–290. - PubMed
-
- Alper MM, Brinsden P, Fischer R and Wikland M (2001) To blastocyst or not to blastocyst? That is the question. Hum Reprod 16:617–619. - PubMed
-
- Antczak M and Van Blerkom J (1999) Temporal and spatial aspects of fragmentation in early human embryos: Possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod 14:429–447. - PubMed
-
- Baart EB, Martini E, van den Berg I, Macklon NS, Galjaard RJ, Fauser BC, et al. (2006) Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum Reprod 21:223–233. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
