Differential Gene Expression and Infection Profiles of Cutaneous and Mucosal Leishmania braziliensis Isolates from the Same Patient
- PMID: 26366580
- PMCID: PMC4569073
- DOI: 10.1371/journal.pntd.0004018
Differential Gene Expression and Infection Profiles of Cutaneous and Mucosal Leishmania braziliensis Isolates from the Same Patient
Abstract
Background: Leishmaniasis is a complex disease in which clinical outcome depends on factors such as parasite species, host genetics and immunity and vector species. In Brazil, Leishmania (Viannia) braziliensis is a major etiological agent of cutaneous (CL) and mucosal leishmaniasis (MCL), a disfiguring form of the disease, which occurs in ~10% of L. braziliensis-infected patients. Thus, clinical isolates from patients with CL and MCL may be a relevant source of information to uncover parasite factors contributing to pathogenesis. In this study, we investigated two pairs of L. (V.) braziliensis isolates from mucosal (LbrM) and cutaneous (LbrC) sites of the same patient to identify factors distinguishing parasites that migrate from those that remain at the primary site of infection.
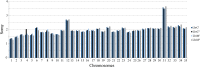
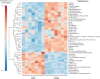
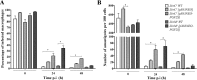
Methodology/principal findings: We observed no major genomic divergences among the clinical isolates by molecular karyotype and genomic sequencing. RT-PCR revealed that the isolates lacked Leishmania RNA virus (LRV). However, the isolates exhibited distinct in vivo pathogenesis in BALB/c mice; the LbrC isolates were more virulent than the LbrM isolates. Metabolomic analysis revealed significantly increased levels of 14 metabolites in LbrC parasites and 31 metabolites in LbrM parasites that were mainly related to inflammation and chemotaxis. A proteome comparative analysis revealed the overexpression of LbrPGF2S (prostaglandin f2-alpha synthase) and HSP70 in both LbrC isolates. Overexpression of LbrPGF2S in LbrC and LbrM promastigotes led to an increase in infected macrophages and the number of amastigotes per cell at 24-48 h post-infection (p.i.).
Conclusions/significance: Despite sharing high similarity at the genome structure and ploidy levels, the parasites exhibited divergent expressed genomes. The proteome and metabolome results indicated differential profiles between the cutaneous and mucosal isolates, primarily related to inflammation and chemotaxis. BALB/c infection revealed that the cutaneous isolates were more virulent than the mucosal parasites. Furthermore, our data suggest that the LbrPGF2S protein is a candidate to contribute to parasite virulence profiles in the mammalian host.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- de Almeida MC, Vilhena V, Barral A, Barral-Netto M. Leishmanial infection: analysis of its first steps. A review. Mem Inst Oswaldo Cruz. 2003;98(7):861–70. - PubMed
-
- Teixeira MJ, Teixeira CR, Andrade BB, Barral-Netto M, Barral A. Chemokines in host-parasite interactions in leishmaniasis. Trends Parasitol. 2006;22(1):32–40. - PubMed
-
- Andrade BB, de Oliveira CI, Brodskyn CI, Barral A, Barral-Netto M. Role of sand fly saliva in human and experimental leishmaniasis: current insights. Scand J Immunol. 2007;66(2–3):122–7. - PubMed
-
- Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581–96. Epub 2007/08/24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

