FANCA safeguards interphase and mitosis during hematopoiesis in vivo
- PMID: 26366677
- PMCID: PMC4666759
- DOI: 10.1016/j.exphem.2015.08.013
FANCA safeguards interphase and mitosis during hematopoiesis in vivo
Abstract
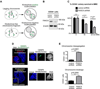
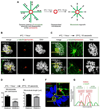
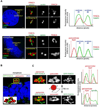
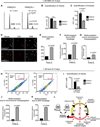
The Fanconi anemia (FA/BRCA) signaling network controls multiple genome-housekeeping checkpoints, from interphase DNA repair to mitosis. The in vivo role of abnormal cell division in FA remains unknown. Here, we quantified the origins of genomic instability in FA patients and mice in vivo and ex vivo. We found that both mitotic errors and interphase DNA damage significantly contribute to genomic instability during FA-deficient hematopoiesis and in nonhematopoietic human and murine FA primary cells. Super-resolution microscopy coupled with functional assays revealed that FANCA shuttles to the pericentriolar material to regulate spindle assembly at mitotic entry. Loss of FA signaling rendered cells hypersensitive to spindle chemotherapeutics and allowed escape from the chemotherapy-induced spindle assembly checkpoint. In support of these findings, direct comparison of DNA crosslinking and anti-mitotic chemotherapeutics in primary FANCA-/- cells revealed genomic instability originating through divergent cell cycle checkpoint aberrations. Our data indicate that FA/BRCA signaling functions as an in vivo gatekeeper of genomic integrity throughout interphase and mitosis, which may have implications for future targeted therapies in FA and FA-deficient cancers.
Copyright © 2015 ISEH - International Society for Experimental Hematology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures



References
-
- Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93(4):511–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

