Overexpression of EcbHLH57 Transcription Factor from Eleusine coracana L. in Tobacco Confers Tolerance to Salt, Oxidative and Drought Stress
- PMID: 26366726
- PMCID: PMC4569372
- DOI: 10.1371/journal.pone.0137098
Overexpression of EcbHLH57 Transcription Factor from Eleusine coracana L. in Tobacco Confers Tolerance to Salt, Oxidative and Drought Stress
Abstract
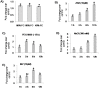
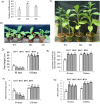
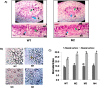
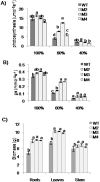
Basic helix-loop-helix (bHLH) transcription factors constitute one of the largest families in plants and are known to be involved in various developmental processes and stress tolerance. We report the characterization of a stress responsive bHLH transcription factor from stress adapted species finger millet which is homologous to OsbHLH57 and designated as EcbHLH57. The full length sequence of EcbHLH57 consisted of 256 amino acids with a conserved bHLH domain followed by leucine repeats. In finger millet, EcbHLH57 transcripts were induced by ABA, NaCl, PEG, methyl viologen (MV) treatments and drought stress. Overexpression of EcbHLH57 in tobacco significantly increased the tolerance to salinity and drought stress with improved root growth. Transgenic plants showed higher photosynthetic rate and stomatal conductance under drought stress that resulted in higher biomass. Under long-term salinity stress, the transgenic plants accumulated higher seed weight/pod and pod number. The transgenic plants were also tolerant to oxidative stress and showed less accumulation of H202 and MDA levels. The overexpression of EcbHLH57 enhanced the expression of stress responsive genes such as LEA14, rd29A, rd29B, SOD, APX, ADH1, HSP70 and also PP2C and hence improved tolerance to diverse stresses.
Conflict of interest statement
Figures


References
-
- Whittaker A, Adriana B, Jill F (2001) Changes in leaf hexokinase activity and metabolite levels in response to drying in the desiccation tolerant species, Sporobolus stapfianus and Xerophyta viscosa . J Exp Bot 52: 961–969. - PubMed
-
- Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Shinozaki K, et al. (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47: 141–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

