Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation
- PMID: 26366848
- PMCID: PMC4654683
- DOI: 10.1038/nmat4407
Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation
Abstract
The effectiveness of stem cell therapies has been hampered by cell death and limited control over fate. These problems can be partially circumvented by using macroporous biomaterials that improve the survival of transplanted stem cells and provide molecular cues to direct cell phenotype. Stem cell behaviour can also be controlled in vitro by manipulating the elasticity of both porous and non-porous materials, yet translation to therapeutic processes in vivo remains elusive. Here, by developing injectable, void-forming hydrogels that decouple pore formation from elasticity, we show that mesenchymal stem cell (MSC) osteogenesis in vitro, and cell deployment in vitro and in vivo, can be controlled by modifying, respectively, the hydrogel's elastic modulus or its chemistry. When the hydrogels were used to transplant MSCs, the hydrogel's elasticity regulated bone regeneration, with optimal bone formation at 60 kPa. Our findings show that biophysical cues can be harnessed to direct therapeutic stem cell behaviours in situ.
Figures

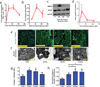

 ) the bulk phase of void-forming hydrogels, or from standard nanoporous hydrogels (▲).The black line denotes the number of cells initially encapsulated into each scaffold. Difference in net cell deployment between the two types of hydrogels was statistically significant (p < 0.01, 2-tailed t-test) at all time-points. (b-c). Kinetics of mMSC deployment from the bulk phase of void-forming hydrogels as a function of porogen degradation rate, as manipulated by controlling (b) the degree of oxidation of polymers used to form porogens (3% (▲), 5% (
) the bulk phase of void-forming hydrogels, or from standard nanoporous hydrogels (▲).The black line denotes the number of cells initially encapsulated into each scaffold. Difference in net cell deployment between the two types of hydrogels was statistically significant (p < 0.01, 2-tailed t-test) at all time-points. (b-c). Kinetics of mMSC deployment from the bulk phase of void-forming hydrogels as a function of porogen degradation rate, as manipulated by controlling (b) the degree of oxidation of polymers used to form porogens (3% (▲), 5% ( ) or 7.5% (
) or 7.5% ( )), or (c) the concentration of calcium (25mM (●), 50mM (
)), or (c) the concentration of calcium (25mM (●), 50mM ( ) or 100mM (
) or 100mM ( )) used to crosslink porogens. Net cell deployment was significantly greater from materials with porogens comprised by 7.5% degree of oxidation (p < 0.001 at all time points after day 0 by Holm-Bonferonni) compared to deployment from materials with either 3 or 5% degree of oxidation. Each degree of porogen crosslinking yielded a level of net deployment that was statistically unique amongst the different materials tested at all time points after day 0 (Holm-Bonferonni test). (d) Analysis of net mMSC deployment at day 7, as a function of the elasticity of bulk component of void-forming gels. Elasticity had a significant effect (1-way ANOVA) on cell deployment. (e). Cumulative cell deployment for mMSC after 1 week of culture in void-forming gels with varying density of RGD peptides. (f). Representative images of Nu/J mice either 7 (top) or 30 (bottom) days after injection of standard (left) or pore-forming hydrogels (right) containing mCherry-expressing mMSC into the subcutaneous tissues of Nu/J mice. (g). Total radiant efficiency (proportional to cell number) from mCherry-mMSC injected within the following hydrogels: void-forming gels with porogens crosslinked with either 100mM (
)) used to crosslink porogens. Net cell deployment was significantly greater from materials with porogens comprised by 7.5% degree of oxidation (p < 0.001 at all time points after day 0 by Holm-Bonferonni) compared to deployment from materials with either 3 or 5% degree of oxidation. Each degree of porogen crosslinking yielded a level of net deployment that was statistically unique amongst the different materials tested at all time points after day 0 (Holm-Bonferonni test). (d) Analysis of net mMSC deployment at day 7, as a function of the elasticity of bulk component of void-forming gels. Elasticity had a significant effect (1-way ANOVA) on cell deployment. (e). Cumulative cell deployment for mMSC after 1 week of culture in void-forming gels with varying density of RGD peptides. (f). Representative images of Nu/J mice either 7 (top) or 30 (bottom) days after injection of standard (left) or pore-forming hydrogels (right) containing mCherry-expressing mMSC into the subcutaneous tissues of Nu/J mice. (g). Total radiant efficiency (proportional to cell number) from mCherry-mMSC injected within the following hydrogels: void-forming gels with porogens crosslinked with either 100mM ( ) or 50mM (
) or 50mM ( ) Ca2+, or within standard hydrogels (▲). Release of cells from either void-forming hydrogel yielded significantly more release than from a standard hydrogel at all time-points beginning at day 10, and altering porogen fabrication yielded a significant effect on radiant efficiency beginning on day 17 (p < 0.05, 2-tailed t-test). RGD density was fixed at 187µM in the bulk gel phase. (h). Total radiant efficiency resulting from mCherry-mMSC injected within void-forming hydrogels in which the RGD concentration was either (
) Ca2+, or within standard hydrogels (▲). Release of cells from either void-forming hydrogel yielded significantly more release than from a standard hydrogel at all time-points beginning at day 10, and altering porogen fabrication yielded a significant effect on radiant efficiency beginning on day 17 (p < 0.05, 2-tailed t-test). RGD density was fixed at 187µM in the bulk gel phase. (h). Total radiant efficiency resulting from mCherry-mMSC injected within void-forming hydrogels in which the RGD concentration was either ( )187µM or (
)187µM or ( ) 750µM within the bulk phase, or within standard hydrogels (▲). Cell transplantation within either void-forming hydrogel type led to substantially higher total radiant efficiency at all time points after day 12 (187µM RGD) or 19 (750µM RGD), compared to transplantation within standard hydrogels. The difference in total radiant efficiency was affected by the density of RGD presented by the bulk phase beginning on day 24 (p < 0.05). (i-j) Representative micrographs of tissues in Nude rat cranial defects one week after transplanting mCherry-mMSC with either i) void-forming or j) standard hydrogels. mCherry antigen was probed with DAB chromogen. Error bars are SEM, n = 3–4 scaffolds (in vitro studies) or n = 4–8 scaffolds (in vivo studies). Scale bar: g,h: 100µm.
) 750µM within the bulk phase, or within standard hydrogels (▲). Cell transplantation within either void-forming hydrogel type led to substantially higher total radiant efficiency at all time points after day 12 (187µM RGD) or 19 (750µM RGD), compared to transplantation within standard hydrogels. The difference in total radiant efficiency was affected by the density of RGD presented by the bulk phase beginning on day 24 (p < 0.05). (i-j) Representative micrographs of tissues in Nude rat cranial defects one week after transplanting mCherry-mMSC with either i) void-forming or j) standard hydrogels. mCherry antigen was probed with DAB chromogen. Error bars are SEM, n = 3–4 scaffolds (in vitro studies) or n = 4–8 scaffolds (in vivo studies). Scale bar: g,h: 100µm.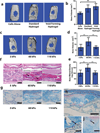
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

