Sparse Spectro-Temporal Receptive Fields Based on Multi-Unit and High-Gamma Responses in Human Auditory Cortex
- PMID: 26367010
- PMCID: PMC4569421
- DOI: 10.1371/journal.pone.0137915
Sparse Spectro-Temporal Receptive Fields Based on Multi-Unit and High-Gamma Responses in Human Auditory Cortex
Abstract
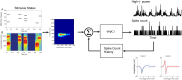
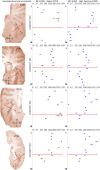
Spectro-Temporal Receptive Fields (STRFs) were estimated from both multi-unit sorted clusters and high-gamma power responses in human auditory cortex. Intracranial electrophysiological recordings were used to measure responses to a random chord sequence of Gammatone stimuli. Traditional methods for estimating STRFs from single-unit recordings, such as spike-triggered-averages, tend to be noisy and are less robust to other response signals such as local field potentials. We present an extension to recently advanced methods for estimating STRFs from generalized linear models (GLM). A new variant of regression using regularization that penalizes non-zero coefficients is described, which results in a sparse solution. The frequency-time structure of the STRF tends toward grouping in different areas of frequency-time and we demonstrate that group sparsity-inducing penalties applied to GLM estimates of STRFs reduces the background noise while preserving the complex internal structure. The contribution of local spiking activity to the high-gamma power signal was factored out of the STRF using the GLM method, and this contribution was significant in 85 percent of the cases. Although the GLM methods have been used to estimate STRFs in animals, this study examines the detailed structure directly from auditory cortex in the awake human brain. We used this approach to identify an abrupt change in the best frequency of estimated STRFs along posteromedial-to-anterolateral recording locations along the long axis of Heschl's gyrus. This change correlates well with a proposed transition from core to non-core auditory fields previously identified using the temporal response properties of Heschl's gyrus recordings elicited by click-train stimuli.
Conflict of interest statement
Figures

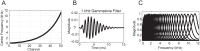




References
-
- Clarke S, Morosan P. Architecture, Connectivity, and Transmitter Receptors of Human Auditory Cortex In: Poeppel D, Overath T, Popper AN, Fay RR, editors. The Human Auditory Cortex. Springer Handbook of Auditory Research. 43: Springer; New York; 2012. p. 11–38.
-
- Howard MA, Volkov IO, Noh MD, Granner MA, Mirsky R, Garell PC. Chronic microelectrode investigations of normal human brain physiology using a hybrid depth electrode. Stereotact Funct Neurosurg. 1997;68(1–4 Pt 1):236–42. Epub 1997/01/01. . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

