The Toll-Like Receptor 5 Agonist Entolimod Mitigates Lethal Acute Radiation Syndrome in Non-Human Primates
- PMID: 26367124
- PMCID: PMC4569586
- DOI: 10.1371/journal.pone.0135388
The Toll-Like Receptor 5 Agonist Entolimod Mitigates Lethal Acute Radiation Syndrome in Non-Human Primates
Abstract
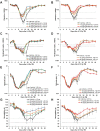
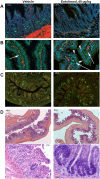
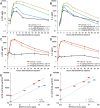
There are currently no approved medical radiation countermeasures (MRC) to reduce the lethality of high-dose total body ionizing irradiation expected in nuclear emergencies. An ideal MRC would be effective even when administered well after radiation exposure and would counteract the effects of irradiation on the hematopoietic system and gastrointestinal tract that contribute to its lethality. Entolimod is a Toll-like receptor 5 agonist with demonstrated radioprotective/mitigative activity in rodents and radioprotective activity in non-human primates. Here, we report data from several exploratory studies conducted in lethally irradiated non-human primates (rhesus macaques) treated with a single intramuscular injection of entolimod (in the absence of intensive individualized supportive care) administered in a mitigative regimen, 1-48 hours after irradiation. Following exposure to LD50-70/40 of radiation, injection of efficacious doses of entolimod administered as late as 25 hours thereafter reduced the risk of mortality 2-3-fold, providing a statistically significant (P<0.01) absolute survival advantage of 40-60% compared to vehicle treatment. Similar magnitude of survival improvement was also achieved with drug delivered 48 hours after irradiation. Improved survival was accompanied by predominantly significant (P<0.05) effects of entolimod administration on accelerated morphological recovery of hematopoietic and immune system organs, decreased severity and duration of thrombocytopenia, anemia and neutropenia, and increased clonogenic potential of the bone marrow compared to control irradiated animals. Entolimod treatment also led to reduced apoptosis and accelerated crypt regeneration in the gastrointestinal tract. Together, these data indicate that entolimod is a highly promising potential life-saving treatment for victims of radiation disasters.
Conflict of interest statement
Figures


References
-
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140(12):1037–51. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

