Sphingosine-1-Phosphate Receptor-1 Selective Agonist Enhances Collateral Growth and Protects against Subsequent Stroke
- PMID: 26367258
- PMCID: PMC4569572
- DOI: 10.1371/journal.pone.0138029
Sphingosine-1-Phosphate Receptor-1 Selective Agonist Enhances Collateral Growth and Protects against Subsequent Stroke
Abstract
Background and purpose: Collateral growth after acute occlusion of an intracranial artery is triggered by increasing shear stress in preexisting collateral pathways. Recently, sphingosine-1-phosphate receptor-1 (S1PR1) on endothelial cells was reported to be essential in sensing fluid shear stress. Here, we evaluated the expression of S1PR1 in the hypoperfused mouse brain and investigated the effect of a selective S1PR1 agonist on leptomeningeal collateral growth and subsequent ischemic damage after focal ischemia.
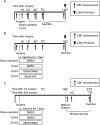
Methods: In C57Bl/6 mice (n = 133) subjected to unilateral common carotid occlusion (CCAO) and sham surgery. The first series examined the time course of collateral growth, cell proliferation, and S1PR1 expression in the leptomeningeal arteries after CCAO. The second series examined the relationship between pharmacological regulation of S1PR1 and collateral growth of leptomeningeal anastomoses. Animals were randomly assigned to one of the following groups: LtCCAO and daily intraperitoneal (i.p.) injection for 7 days of an S1PR1 selective agonist (SEW2871, 5 mg/kg/day); sham surgery and daily i.p. injection for 7 days of SEW2871 after surgery; LtCCAO and daily i.p. injection for 7 days of SEW2871 and an S1PR1 inverse agonist (VPC23019, 0.5 mg/kg); LtCCAO and daily i.p. injection of DMSO for 7 days after surgery; and sham surgery and daily i.p. injection of DMSO for 7 days. Leptomeningeal anastomoses were visualized 14 days after LtCCAO by latex perfusion method, and a set of animals underwent subsequent permanent middle cerebral artery occlusion (pMCAO) 7 days after the treatment termination. Neurological functions 1 hour, 1, 4, and 7 days and infarction volume 7 days after pMCAO were evaluated.
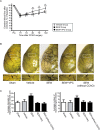
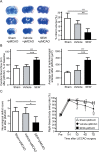
Results: In parallel with the increase in S1PR1 mRNA levels, S1PR1 expression colocalized with endothelial cell markers in the leptomeningeal arteries, increased markedly on the side of the CCAO, and peaked 7 days after CCAO. Mitotic cell numbers in the leptomeningeal arteries increased after CCAO. Administration of the S1PR1 selective agonist significantly increased cerebral blood flow (CBF) and the diameter of leptomeningeal collateral vessels (42.9 ± 2.6 μm) compared with the controls (27.6 ± 5.7 μm; P < 0.01). S1PR1 inverse agonist administration diminished the effect of the S1PR1 agonist (P < 0.001). After pMCAO, S1PR1 agonist pretreated animals showed significantly smaller infarct volume (17.5% ± 4.0% vs. 7.7% ± 4.0%, P < 0.01) and better functional recovery than vehicle-treated controls.
Conclusions: These results suggest that S1PR1 is one of the principal regulators of leptomeningeal collateral recruitment at the site of increased shear stress and provide evidence that an S1PR1 selective agonist has a role in promoting collateral growth and preventing of ischemic damage and neurological dysfunction after subsequent stroke in patients with intracranial major artery stenosis or occlusion.
Conflict of interest statement
Figures



Similar articles
-
Sphingosine-1-Phosphate Receptor 1 Activation Enhances Leptomeningeal Collateral Development and Improves Outcome after Stroke in Mice.J Stroke Cerebrovasc Dis. 2018 May;27(5):1237-1251. doi: 10.1016/j.jstrokecerebrovasdis.2017.11.040. Epub 2018 Jan 11. J Stroke Cerebrovasc Dis. 2018. PMID: 29337049
-
Sinusoidal protection by sphingosine-1-phosphate receptor 1 agonist in liver ischemia-reperfusion injury.J Surg Res. 2018 Feb;222:139-152. doi: 10.1016/j.jss.2017.09.048. Epub 2017 Nov 4. J Surg Res. 2018. PMID: 29273365
-
Progressive Disruption of Sphingosine-1-Phosphate Receptor 1 Correlates with Blood-Brain Barrier Leakage in A Rat Model of Chronic Hypoxic Hypoperfusion.Aging Dis. 2024 May 21;16(2):1099-1119. doi: 10.14336/AD.2024.0098. Aging Dis. 2024. PMID: 38916732 Free PMC article.
-
Acute development of collateral circulation and therapeutic prospects in ischemic stroke.Neural Regen Res. 2016 Mar;11(3):368-71. doi: 10.4103/1673-5374.179033. Neural Regen Res. 2016. PMID: 27127459 Free PMC article. Review.
-
Leptomeningeal anastomoses: Mechanisms of pial collateral remodeling in ischemic stroke.WIREs Mech Dis. 2022 Jul;14(4):e1553. doi: 10.1002/wsbm.1553. Epub 2022 Feb 3. WIREs Mech Dis. 2022. PMID: 35118835 Free PMC article. Review.
Cited by
-
Differences in collateral vessel formation after experimental retinal vein occlusion in spontaneously hypertensive rats and wild-type rats.Heliyon. 2024 Mar 1;10(6):e27160. doi: 10.1016/j.heliyon.2024.e27160. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38509953 Free PMC article.
-
Promising Cerebral Blood Flow Enhancers in Acute Ischemic Stroke.Transl Stroke Res. 2023 Dec;14(6):863-889. doi: 10.1007/s12975-022-01100-w. Epub 2022 Nov 17. Transl Stroke Res. 2023. PMID: 36394792 Free PMC article. Review.
-
Enhanced Phospholipase A2 Group 3 Expression by Oxidative Stress Decreases the Insulin-Degrading Enzyme.PLoS One. 2015 Dec 4;10(12):e0143518. doi: 10.1371/journal.pone.0143518. eCollection 2015. PLoS One. 2015. PMID: 26637123 Free PMC article.
-
Adipose tissue and metabolic and inflammatory responses to stroke are altered in obese mice.Dis Model Mech. 2017 Oct 1;10(10):1229-1243. doi: 10.1242/dmm.030411. Epub 2017 Aug 10. Dis Model Mech. 2017. PMID: 28798136 Free PMC article.
-
Preferential delivery of lipid-ligand conjugated DNA/RNA heteroduplex oligonucleotide to ischemic brain in hyperacute stage.Mol Ther. 2023 Apr 5;31(4):1106-1122. doi: 10.1016/j.ymthe.2023.01.016. Epub 2023 Jan 24. Mol Ther. 2023. PMID: 36694463 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

