Sphingosine-1-Phosphate Signaling Regulates Myogenic Responsiveness in Human Resistance Arteries
- PMID: 26367262
- PMCID: PMC4569583
- DOI: 10.1371/journal.pone.0138142
Sphingosine-1-Phosphate Signaling Regulates Myogenic Responsiveness in Human Resistance Arteries
Abstract
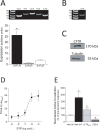
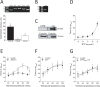
We recently identified sphingosine-1-phosphate (S1P) signaling and the cystic fibrosis transmembrane conductance regulator (CFTR) as prominent regulators of myogenic responsiveness in rodent resistance arteries. However, since rodent models frequently exhibit limitations with respect to human applicability, translation is necessary to validate the relevance of this signaling network for clinical application. We therefore investigated the significance of these regulatory elements in human mesenteric and skeletal muscle resistance arteries. Mesenteric and skeletal muscle resistance arteries were isolated from patient tissue specimens collected during colonic or cardiac bypass surgery. Pressure myography assessments confirmed endothelial integrity, as well as stable phenylephrine and myogenic responses. Both human mesenteric and skeletal muscle resistance arteries (i) express critical S1P signaling elements, (ii) constrict in response to S1P and (iii) lose myogenic responsiveness following S1P receptor antagonism (JTE013). However, while human mesenteric arteries express CFTR, human skeletal muscle resistance arteries do not express detectable levels of CFTR protein. Consequently, modulating CFTR activity enhances myogenic responsiveness only in human mesenteric resistance arteries. We conclude that human mesenteric and skeletal muscle resistance arteries are a reliable and consistent model for translational studies. We demonstrate that the core elements of an S1P-dependent signaling network translate to human mesenteric resistance arteries. Clear species and vascular bed variations are evident, reinforcing the critical need for further translational study.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

