Intrinsic MyD88-Akt1-mTOR Signaling Coordinates Disparate Tc17 and Tc1 Responses during Vaccine Immunity against Fungal Pneumonia
- PMID: 26367276
- PMCID: PMC4569330
- DOI: 10.1371/journal.ppat.1005161
Intrinsic MyD88-Akt1-mTOR Signaling Coordinates Disparate Tc17 and Tc1 Responses during Vaccine Immunity against Fungal Pneumonia
Abstract
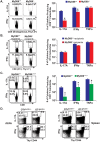
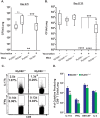
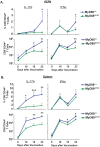
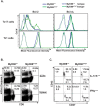
Fungal infections have skyrocketed in immune-compromised patients lacking CD4+ T cells, underscoring the need for vaccine prevention. An understanding of the elements that promote vaccine immunity in this setting is essential. We previously demonstrated that vaccine-induced IL-17A+ CD8+ T cells (Tc17) are required for resistance against lethal fungal pneumonia in CD4+ T cell-deficient hosts, whereas the individual type I cytokines IFN-γ, TNF-α and GM-CSF, are dispensable. Here, we report that T cell-intrinsic MyD88 signals are crucial for these Tc17 cell responses and vaccine immunity against lethal fungal pneumonia in mice. In contrast, IFN-γ+ CD8+ cell (Tc1) responses are largely normal in the absence of intrinsic MyD88 signaling in CD8+ T cells. The poor accumulation of MyD88-deficient Tc17 cells was not linked to an early onset of contraction, nor to accelerated cell death or diminished expression of anti-apoptotic molecules Bcl-2 or Bcl-xL. Instead, intrinsic MyD88 was required to sustain the proliferation of Tc17 cells through the activation of mTOR via Akt1. Moreover, intrinsic IL-1R and TLR2, but not IL-18R, were required for MyD88 dependent Tc17 responses. Our data identify unappreciated targets for augmenting adaptive immunity against fungi. Our findings have implications for designing fungal vaccines and immune-based therapies in immune-compromised patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
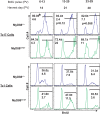
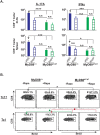
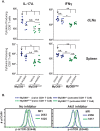

Similar articles
-
Antifungal Tc17 cells are durable and stable, persisting as long-lasting vaccine memory without plasticity towards IFNγ cells.PLoS Pathog. 2017 May 22;13(5):e1006356. doi: 10.1371/journal.ppat.1006356. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28542595 Free PMC article.
-
Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells.PLoS Pathog. 2012;8(7):e1002771. doi: 10.1371/journal.ppat.1002771. Epub 2012 Jul 19. PLoS Pathog. 2012. PMID: 22829762 Free PMC article.
-
MyD88 Shapes Vaccine Immunity by Extrinsically Regulating Survival of CD4+ T Cells during the Contraction Phase.PLoS Pathog. 2016 Aug 19;12(8):e1005787. doi: 10.1371/journal.ppat.1005787. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27542117 Free PMC article.
-
MyDths and un-TOLLed truths: sensor, instructive and effector immunity to tuberculosis.Immunol Lett. 2008 Feb 15;116(1):15-23. doi: 10.1016/j.imlet.2007.11.015. Epub 2007 Dec 26. Immunol Lett. 2008. PMID: 18191460 Review.
-
Tc17 Cells in Immunity and Systemic Autoimmunity.Int Rev Immunol. 2015;34(4):318-31. doi: 10.3109/08830185.2014.954698. Epub 2014 Sep 26. Int Rev Immunol. 2015. PMID: 25259411 Review.
Cited by
-
Antifungal Tc17 cells are durable and stable, persisting as long-lasting vaccine memory without plasticity towards IFNγ cells.PLoS Pathog. 2017 May 22;13(5):e1006356. doi: 10.1371/journal.ppat.1006356. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28542595 Free PMC article.
-
Toll-Like Receptor 2 Mediates In Vivo Pro- and Anti-inflammatory Effects of Mycobacterium Tuberculosis and Modulates Autoimmune Encephalomyelitis.Front Immunol. 2016 May 24;7:191. doi: 10.3389/fimmu.2016.00191. eCollection 2016. Front Immunol. 2016. PMID: 27252700 Free PMC article.
-
Helper T-cell responses and pulmonary fungal infections.Immunology. 2018 Oct;155(2):155-163. doi: 10.1111/imm.12953. Epub 2018 Jun 14. Immunology. 2018. PMID: 29781185 Free PMC article. Review.
-
Long-Term Accumulation of T Cytotoxic 1, T Cytotoxic 17, and T Cytotoxic 17/1 Cells in the Brain Contributes to Microglia-Mediated Chronic Neuroinflammation After Ischemic Stroke.Neuromolecular Med. 2024 Apr 29;26(1):17. doi: 10.1007/s12017-024-08786-1. Neuromolecular Med. 2024. PMID: 38684592
-
Histone deacetylase (HDAC) inhibitor ACY241 enhances anti-tumor activities of antigen-specific central memory cytotoxic T lymphocytes against multiple myeloma and solid tumors.Leukemia. 2018 Sep;32(9):1932-1947. doi: 10.1038/s41375-018-0062-8. Epub 2018 Feb 22. Leukemia. 2018. PMID: 29487385 Free PMC article.
References
-
- Seder RA, Ahmed R (2003) Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol 4: 835–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

