Type I interferons for induction of remission in ulcerative colitis
- PMID: 26368001
- PMCID: PMC9196197
- DOI: 10.1002/14651858.CD006790.pub3
Type I interferons for induction of remission in ulcerative colitis
Abstract
Background: Interferons (IFNs) are cytokines which possess immunoregulatory properties and have been used to successfully treat a number of chronic inflammatory disorders. It has been postulated that Type I IFNs may be able to re-establish the Th1/Th2 balance in Th2 predominant diseases like ulcerative colitis.
Objectives: To systematically evaluate the efficacy and safety of type I IFN therapy for induction of remission in ulcerative colitis.
Search methods: We searched MEDLINE, EMBASE, CENTRAL, the Cochrane IBD/FBD group specialised register, and ClinicalTrials.gov from inception to August 8, 2014. Reference lists of trials and review articles, as well as recent proceedings from major gastroenterology meetings were manually searched.
Selection criteria: Randomised controlled trials of type I IFNs for induction of remission in UC were included. The study population included patients of any age with active ulcerative colitis. There were no exclusions based on type, dose or duration of IFN treatment.
Data collection and analysis: Two independent authors reviewed studies for eligibility, extracted the data and assessed study quality using the Cochrane risk of bias tool. The overall quality of the evidence supporting the outcomes was evaluated using the GRADE criteria. The primary outcome was induction of remission of ulcerative colitis. Secondary outcomes included: time to remission, mean change in disease activity index score, clinical, histological or endoscopic improvement, improvement in quality of life, and adverse events. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for dichotomous outcomes. We calculated the mean difference and corresponding 95% confidence interval for continuous outcomes. Meta-analysis was performed using RevMan 5.3.5 software.
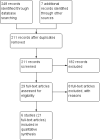
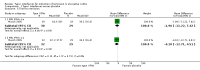
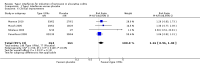
Main results: Six studies were eligible for inclusion (517 patients). Five studies compared type I IFNs to placebo injections (485 patients) and a single study compared IFNs to prednisolone enemas in patients with left-sided colitis (32 patients). The active comparator study was rated as high risk of bias due to an open-label design. Three studies were rated as unclear risk of bias for random sequence generation and allocation concealment. Two studies described as double blind were rated as unclear risk of bias for blinding. There was no significant benefit of type I IFNs over placebo for inducing clinical remission or improvement in patients with active ulcerative colitis. Thirty-six per cent (87/242) of patients in the type I IFNs group achieved clinical remission by 8 to 12 weeks compared to 30% (36/120) of placebo patients (RR 1.16, 95% CI 0.84 to 1.58; 4 studies, 362 patients). A GRADE analysis indicated that the overall quality of the evidence supporting the outcome clinical remission was moderate due to sparse data (123 events). Fifty-six per cent (149/264) of patients in the type I IFNs group improved clinically by 8 to 12 weeks compared to 48% (77/161) of placebo patients (RR 1.16, 95% CI 0.96 to 1.40; 4 studies, 425 patients). A GRADE analysis indicated that the overall quality of the evidence supporting the outcome clinical improvement was moderate due to sparse data (226 events). Patients who received type I IFNs were significantly more likely to withdraw from the studies due to adverse events than those who received placebo. Seven per cent (18/42) of type I IFNs patients withdrew due to adverse events compared to 2% (3/152) of placebo patients (RR 3.16, 95% CI 1.06 to 9.40). A GRADE analysis indicated that the overall quality of the evidence supporting the outcome withdrawal due to adverse events was low due to very sparse data (21 events). The study comparing type I IFNs to prednisolone enemas found no difference between the treatment groups in quality of life or disease activity scores. Common adverse events included headaches, arthralgias, myalgias, fatigue, back pain, nausea, application site reactions, rigors, and fevers. There were no statistically significant differences in the other secondary outcomes.
Authors' conclusions: Moderate quality evidence suggests that type I IFNs are not effective for the induction of remission in UC. In addition, there are concerns regarding the tolerability of this class of treatment.
Conflict of interest statement
YW: None known.
JKM: None known.
EIB: None known.
AMG: Anne Marie Griffiths has received fee(s) from Johnson and Johnson for Board membership; fee(s) from Janssen, Abbvie and Ferring for consultancy; grants or grants pending from Johnson and Johnson and Abbive; lecture fee(s) from: Abbvie and Merck and payment for development of educational presentations from Ferring. All of these activities are outside the submitted work.
AHS: Hillary Steinhart has received fee(s) from Janssen, Abbvie, Shire, Pendopharm, Pfizer, and Takeda for consultancy; and lecture fee(s) from: Janssen, Abbvie, Shire, Warner Chilcott, Aptalis, and Takeda. His institution has received grants or grants pending from Janssen, Abbvie, Pfizer, Amgen, Takeda and Actavis. All of these activities are outside the submitted work. AHS was a collaborator on the paper "Interferon β‐1a in ulcerative colitis: a placebo controlled, randomised, dose escalating study." (Gut 2003;52:1286‐1290) and Mount Sinai Hospital, Toronto, Ontario, CANADA was one of the participating study centres in the clinical trial.
RP: Remo Panaccione has received fee(s) from Abbott, Abbvie, Allergan, Actavis, Biogen Idec, Celgene, Eisai, Elan, Ferring, Genentech, Janssen, Merck, Nestle, Osiris, Prometheus, Qu Biologics, Roche, Salix, Takeda, Teva, Vertex, Warner Chilcott for consultancy; grants or grants pending from Abbvie, Janssen; lecture fee(s) from: Abbott, Abbvie, Ferring, Janssen, Shire, Takeda; and payment for development of educational presentations from Abbvie, Janssen, Takeda, Shire. All of these activities are outside the submitted work. RP was a collaborator on the paper "Interferon‐ß‐1a in active moderate to severe ulcerative colitis: Efficacy and safety from a phase IIa multicenter study." (American Journal of Gastroenterology 2010;105:S446).
CHS: Cynthia Seow has served as a consultant and on advisory boards for Janssen Pharmaceuticals, Abbvie, Takeda, Shire and Actavis. She previously held a grant through Janssen Pharmaceuticals. Dr. Seow has also provided lectures for Janssen Pharmaceuticals and Warner Chilcott. All of these activities are outside the submitted work.
Figures













Update of
-
Type I interferons for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD006790. doi: 10.1002/14651858.CD006790.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2015 Sep 14;(9):CD006790. doi: 10.1002/14651858.CD006790.pub3. PMID: 18646167 Updated.
References
References to studies included in this review
Madsen 2001 {published data only}
-
- Madsen SM, Schlichting P, Davidsen B, Nielsen OH, Federspiel B, Riis P, et al. An open, randomized study comparing systemic interferon‐a‐2A and prednisolone enemas in the treatment of left‐sided ulcerative colitis. Gut 1999;45(Suppl V):A286. - PubMed
-
- Madsen SM, Schlichting P, Davidsen B, Nielsen OH, Federspiel B, Riis P, et al. An open‐labeled, randomized study comparing systemic interferon‐alpha‐2A and prednisolone enemas in the treatment of left‐sided ulcerative colitis. American Journal of Gastroenterology 2001;96(6):1807‐15. - PubMed
Mannon 2010 {published data only}
-
- Mannon P, Feagan B, Xi Y, McAllister A, Cataldi F. Mucosal healing an essential efficacy measure in ulcerative colitis: Effect of country‐enrollment site in a phase IIa study with interferon‐ß‐1a. Journal of Crohn's and Colitis 2011;5(1):S102‐3.
-
- Mannon P, Miner P, Lukas M, McAllister A, Cataldi F. Secondary efficacy analysis from a phase IIa multicenter study with interferon‐ß‐1a for induction of clinical response in active moderate to severe ulcerative colitis. American Journal of Gatroenterology 2010;105:S468‐9.
-
- Mannon P, Panaccione R, Miner P, McAllister A, O'Gorman J, Cataldi F. Interferon‐ß‐1a in active moderate to severe ulcerative colitis: Efficacy and safety from a phase IIa multicenter study. American Journal of Gastroenterology 2010;105:S446.
-
- Mannon P, Reinisch W, Miner P, McAllister A, Xi Y, Cataldi F. Different scoring methods affect the accuracy of clinical outcomes in ulcerative colitis. Post‐hoc analysis in a phase 2a study with interferon‐ß‐1a. Journal of Crohn's and Colitis 2011;5(1):S69‐70.
-
- Mannon P, Reinisch W, Miner P, Xi Y, McAllister A, Cataldi F. Are the results of multicenter trials hampered by country variability? Comparison between two endpoints used in a study with interferon‐ß‐1a in ulcerative colitis. Journal of Crohn's and Colitis 2011;5(1):S94‐5.
Musch 2005 {published data only}
-
- Musch E, Andus T, Kruis W, Raedler A, Spehlmann M, Schreiber S, et al. Interferon‐beta‐1a for the treatment of steroid‐refractory ulcerative colitis: a randomized, double‐blind, placebo‐controlled trial. Clinical Gastroenterology and Hepatology 2005;3(6):581‐6. - PubMed
-
- Musch E, Raedler A, Andus T, Kruis W, Schreiber S, Lorenz A, et al. A phase II placebo‐controlled, randomized multicenter study to evaluate efficacy and safety of interferon beta‐1a in patients with ulcerative colitis. Gastroenterology 2002;122(4 Suppl 1):A431.
-
- Musch E, Raedler A, Andus T, Kruis W, Schreiber S, Lorenz A, et al. Results of a randomised, double‐blind, placebo‐controlled, multi‐centre, phase‐II study to test the effectivity and tolerance of Interferon‐beta‐1a (CHO‐beta) [rIFN‐beta‐1a] in patients with ulcerative colitis. Zeitschrift fur Gastroenterologie 2002;40(8):705.
Nikolaus 2003 {published data only}
-
- Nikolaus S, Rutgeerts P, Fedorak RN, Steinhart H, Wild GE, Theuer D, et al. Recombinant human interferon‐beta (IFNb‐1a) induces remission and is well tolerated in moderately active ulcerative colitis (UC). Gastroenterology 2001;120(5 Suppl 1):A454.
Pena‐Rossi 2008 {published data only}
-
- Pena‐Rossi C, Schreiber S, Golubovic G, Mertz‐Nielsen A, Panes J, Rachmilewitz D, et al. Clinical trial: a multicentre, randomized, double‐blind, placebo‐controlled, dose‐finding, phase II study of subcutaneous interferon‐beta‐la in moderately active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2008;28(6):758‐67. - PubMed
Tilg 2003 {published data only}
References to studies excluded from this review
Bargiggia 2005 {published data only}
-
- Bargiggia S, Thorburn D, Anderloni A, Ardizzone S, Giorgi A, Porro GB, et al. Is interferon‐alpha therapy safe and effective for patients with chronic hepatitis C and inflammatory bowel disease? A case‐control study. Alimentary Pharmacology and Therapeutics 2005;22(3):209‐15. - PubMed
Hadziselimovic 1995 {published data only}
-
- Hadziselimovic F, Schaub U, Emmons LR. Interferon alpha‐2A (roferon) as a treatment of inflammatory bowel disease in children and adolescents. Advances in Experimental Medicine and Biology 1995;371B:1323‐6. - PubMed
Maev 2002 {published data only}
-
- Maev IV, Grigorian SS, Gadzhieva MG, Ovchinnikova NI, Ospel'nikova TP, Kaziulin AN. [Interferon status of patients with non‐specific ulcerous colitis and its correction with interferon inducers]. Terapevticheskiĭ Arkhiv 2002;74(2):31‐5. - PubMed
Mannon 2011 {published data only}
-
- Cataldi F, Mannon P, Fuss I, Groden C, Strober W. A phase IIa study of AVONEX (interferon‐B‐1a) in ulcerative colitis (UC): Integrating basic immunology and human pilot study results. Journal of Crohn's and Colitis Supplements 2010;4(1):33.
Musch 2002 {published data only}
-
- Musch E, Andus T, Malek M. Induction and maintenance of clinical remission by interferon‐beta in patients with steroid‐refractory active ulcerative colitis ‐ an open long‐term pilot trial. Alimentary Pharmacology and Therapeutics 2002;16(7):1233‐9. - PubMed
Musch 2007 {published data only}
-
- Musch E, Andus T, Malek M, Chrissafidou A, Schulz M. Successful treatment of steroid refractory active ulcerative colitis with natural interferon‐beta‐‐an open long‐term trial. Zeitschrift für Gastroenterologie 2007;45(12):1235‐40. - PubMed
Sümer 1995 {published data only}
-
- Sümer N, Palabiyikoğlu M. Induction of remission by interferon‐alpha in patients with chronic active ulcerative colitis. European Journal of Gastroenterology and Hepatology 1995;7(7):597‐602. - PubMed
Additional references
Anonymous 1998
-
- Anonymous. Randomised double‐blind placebo‐controlled study of interferon beta‐1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta‐1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998;352(9139):1498‐504. - PubMed
Anonymous 2001
-
- Anonymous. PRISMS‐4: Long‐term efficacy of interferon‐beta‐1a in relapsing MS. Neurology 2001;56(12):1628‐36. - PubMed
Antonetti 2002
-
- Antonetti F, Finocchiaro O, Mascia M, Terlizzese MG, Jaber A. A comparison of the biologic activity of two recombinant IFN‐beta preparations used in the treatment of relapsing‐remitting multiple sclerosis. Journal of Interferon & Cytokine Research 2002;22(12):1181‐4. - PubMed
Baumgart 2007
-
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet 2007;369(9573):1627‐40. - PubMed
Beattie 1996
-
- Beattie RM, Nicholls SW, Domizio P, Williams CB, Walker‐Smith JA. Endoscopic assessment of the colonic response to corticosteroids in children with ulcerative colitis. Journal of Pediatric Gastroenterology and Nutrition 1996;22(4):373‐9. - PubMed
Bernstein 2015
-
- Bernstein CN. Treatment of IBD: where we are and where we are going. American Journal of Gastroenterology 2015;110(1):114‐26. - PubMed
Borg 2007
-
- Borg FA, Isenberg DA. Syndromes and complications of interferon therapy. Current Opinion in Rheumatology 2007;19(1):61‐6. - PubMed
Bouma 2003
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature reviews. Immunology 2003;3(7):521‐33. - PubMed
Brassard 2002
-
- Brassard DL, Grace MJ, Bordens RW. Interferon‐alpha as an immunotherapeutic protein. Journal of Leukocyte Biology 2002;71(4):565‐81. - PubMed
Durbin 2013
Egger 1997
Ek 2014
Feagan 2013
-
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699‐710. - PubMed
Follmann 1992
-
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. - PubMed
Foster 2004
-
- Foster GR. Review article: pegylated interferons: chemical and clinical differences. Alimentary Pharmacology and Therapeutics 2004;20(8):825‐30. - PubMed
George 2012
-
- George PM, Badiger R, Alazawi W, Foster GR, Mitchell JA. Pharmacology and therapeutic potential of interferons. Pharmacology & Therapeutics 2012;135(1):44‐53. - PubMed
Geremia 2014
-
- Geremia A, Biancheri P, Allan P, Corazza GR, Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmunity Reviews 2014;13(1):3‐10. - PubMed
Ghosh 2006
Graber 2007
Guyatt 1989
-
- Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96(3):804‐10. - PubMed
Guyatt 2008
Hanauer 2006
-
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflammatory Bowel Diseases 2006;12 Suppl 1:S3‐9. - PubMed
Hansen 2010
Heller 2005
-
- Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin‐13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005;129(2):550‐64. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoofnagle 2006
-
- Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. New England Journal of Medicine 2006;355(23):2444‐51. - PubMed
Ivashkiv 2014
Jovanovic 2014
Kasper 2014
Ko 2014
Koretz 2013
Lichtiger 1990
-
- Lichtiger S, Present DH. Preliminary report: cyclosporin in treatment of severe active ulcerative colitis. Lancet 1990;336(8706):16‐9. - PubMed
Ludigs 2012
Mavrogiannis 2001
-
- Mavrogiannis C, Papanikolaou IS, Elefsiniotis IS, Psilopoulos DI, Karameris A, Karvountzis G. Ulcerative colitis associated with interferon treatment for chronic hepatitis C. Journal of Hepatology 2001;34(6):964‐5. - PubMed
Okanoue 1996
-
- Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, et al. Side effects of high‐dose interferon therapy for chronic hepatitis C. Journal of Hepatology 1996;25(3):283‐91. - PubMed
Powell‐Tuck 1978
-
- Powell‐Tuck J, Bown RL, Lennard‐Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scandinavian Journal of Gastroenterology 1978;13(7):833‐7. - PubMed
Rachmilewitz 1989
Rutgeerts 2005
-
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2005;353(23):2462‐76. - PubMed
Schroeder 1987
-
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. New England Journal of Medicine 1987;317(26):1625‐9. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Seo 1992
-
- Seo M, Okada M, Yao T, Ueki M, Arima S, Okumura M. An index of disease activity in patients with ulcerative colitis. American Journal of Gastroenterology 1992;87(8):971‐6. - PubMed
Shibuya 2005
-
- Shibuya H, Hirohata S. Differential effects of IFN‐alpha on the expression of various TH2 cytokines in human CD4+ T cells. Journal of Allergy and Clinical Immunology 2005;116(1):205‐12. - PubMed
Sparano 1991
-
- Sparano JA, Dutcher JP, Kaleya R, Caliendo G, Fiorito J, Mitsudo S, et al. Colonic ischemia complicating immunotherapy with interleukin‐2 and interferon‐alpha. Cancer 1991;68(7):1538‐44. - PubMed
Sprenger 2005
Tada 1996
-
- Tada H, Saitoh S, Nakagawa Y, Hirana H, Morimoto M, Shima T, et al. Ischemic colitis during interferon‐alpha treatment for chronic active hepatitis C. Journal of Gastroenterology 1996;31(4):582‐4. - PubMed
Timmer 2012
Travis 2006
-
- Travis SP. Review article: induction therapy for patients with active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;24 Suppl 1:10‐6. - PubMed
Travis 2012
Truelove 1955
Tsukada 2002
-
- Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. American Journal of Gastroenterology 2002;97(11):2820‐8. - PubMed
Tursi 2007
-
- Tursi A. Rapid onset of ulcerative colitis after treatment with PEG‐interferon plus ribavirin for chronic hepatitis C. Inflammatory Bowel Diseases 2007;13(9):1189‐90. - PubMed
Walmsley 1998
Watanabe 2006
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

