Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis
- PMID: 26368307
- PMCID: PMC4607113
- DOI: 10.1172/JCI79271
Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis
Abstract
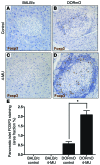
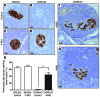
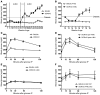
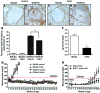
We recently reported that abundant deposits of the extracellular matrix polysaccharide hyaluronan (HA) are characteristic of autoimmune insulitis in patients with type 1 diabetes (T1D), but the relevance of these deposits to disease was unclear. Here, we have demonstrated that HA is critical for the pathogenesis of autoimmune diabetes. Using the DO11.10xRIPmOVA mouse model of T1D, we determined that HA deposits are temporally and anatomically associated with the development of insulitis. Moreover, treatment with an inhibitor of HA synthesis, 4-methylumbelliferone (4-MU), halted progression to diabetes even after the onset of insulitis. Similar effects were seen in the NOD mouse model, and in these mice, 1 week of treatment was sufficient to prevent subsequent diabetes. 4-MU reduced HA accumulation, constrained effector T cells to nondestructive insulitis, and increased numbers of intraislet FOXP3+ Tregs. Consistent with the observed effects of 4-MU treatment, Treg differentiation was inhibited by HA and anti-CD44 antibodies and rescued by 4-MU in an ERK1/2-dependent manner. These data may explain how peripheral immune tolerance is impaired in tissues under autoimmune attack, including islets in T1D. We propose that 4-MU, already an approved drug used to treat biliary spasm, could be repurposed to prevent, and possibly treat, T1D in at-risk individuals.
Figures




References
-
- Glisic S, Ehlenbach S, Jailwala P, Waukau J, Jana S, Ghosh S. Inducible regulatory T cells (iTregs) from recent-onset type 1 diabetes subjects show increased in vitro suppression and higher ITCH levels compared with controls. Cell Tissue Res. 2010;339(3):585–595. doi: 10.1007/s00441-009-0900-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

