Impact-Free Measurement of Microtubule Rotations on Kinesin and Cytoplasmic-Dynein Coated Surfaces
- PMID: 26368807
- PMCID: PMC4569553
- DOI: 10.1371/journal.pone.0136920
Impact-Free Measurement of Microtubule Rotations on Kinesin and Cytoplasmic-Dynein Coated Surfaces
Abstract
Knowledge about the three-dimensional stepping of motor proteins on the surface of microtubules (MTs) as well as the torsional components in their power strokes can be inferred from longitudinal MT rotations in gliding motility assays. In previous studies, optical detection of these rotations relied on the tracking of rather large optical probes present on the outer MT surface. However, these probes may act as obstacles for motor stepping and may prevent the unhindered rotation of the gliding MTs. To overcome these limitations, we devised a novel, impact-free method to detect MT rotations based on fluorescent speckles within the MT structure in combination with fluorescence-interference contrast microscopy. We (i) confirmed the rotational pitches of MTs gliding on surfaces coated by kinesin-1 and kinesin-8 motors, (ii) demonstrated the superiority of our method over previous approaches on kinesin-8 coated surfaces at low ATP concentration, and (iii) identified MT rotations driven by mammalian cytoplasmic dynein, indicating that during collective motion cytoplasmic dynein side-steps with a bias in one direction. Our novel method is easy to implement on any state-of-the-art fluorescence microscope and allows for high-throughput experiments.
Conflict of interest statement
Figures
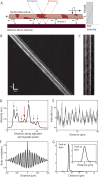

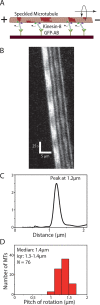
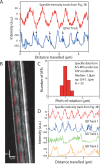


Similar articles
-
Activation of mammalian cytoplasmic dynein in multimotor motility assays.J Cell Sci. 2018 Nov 26;132(4):jcs220079. doi: 10.1242/jcs.220079. J Cell Sci. 2018. PMID: 30237223
-
Quantum-dot-assisted characterization of microtubule rotations during cargo transport.Nat Nanotechnol. 2008 Sep;3(9):552-6. doi: 10.1038/nnano.2008.216. Epub 2008 Aug 10. Nat Nanotechnol. 2008. PMID: 18772917
-
Bidirectional helical motility of cytoplasmic dynein around microtubules.Elife. 2014 Jul 28;3:e03205. doi: 10.7554/eLife.03205. Elife. 2014. PMID: 25069614 Free PMC article.
-
Fluorescence microscopy applied to intracellular transport by microtubule motors.J Biosci. 2018 Jul;43(3):437-445. J Biosci. 2018. PMID: 30002263 Review.
-
Role of cytoplasmic dynein and kinesins in adenovirus transport.FEBS Lett. 2020 Jun;594(12):1838-1847. doi: 10.1002/1873-3468.13777. Epub 2020 Apr 13. FEBS Lett. 2020. PMID: 32215924 Review.
Cited by
-
A Brownian Ratchet Model Explains the Biased Sidestepping of Single-Headed Kinesin-3 KIF1A.Biophys J. 2019 Jun 18;116(12):2266-2274. doi: 10.1016/j.bpj.2019.05.011. Epub 2019 May 18. Biophys J. 2019. PMID: 31155147 Free PMC article.
-
Mechanisms of Regulation in Intraflagellar Transport.Cells. 2022 Sep 2;11(17):2737. doi: 10.3390/cells11172737. Cells. 2022. PMID: 36078145 Free PMC article. Review.
-
CYK4 relaxes the bias in the off-axis motion by MKLP1 kinesin-6.Commun Biol. 2021 Feb 10;4(1):180. doi: 10.1038/s42003-021-01704-2. Commun Biol. 2021. PMID: 33568771 Free PMC article.
-
Kinesin-14 motors drive a right-handed helical motion of antiparallel microtubules around each other.Nat Commun. 2020 May 22;11(1):2565. doi: 10.1038/s41467-020-16328-z. Nat Commun. 2020. PMID: 32444784 Free PMC article.
-
3D motion of vesicles along microtubules helps them to circumvent obstacles in cells.J Cell Sci. 2017 Jun 1;130(11):1904-1916. doi: 10.1242/jcs.201178. Epub 2017 Apr 18. J Cell Sci. 2017. PMID: 28420672 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

