Identification of Tetraazacyclic Compounds as Novel Potent Inhibitors Antagonizing RORγt Activity and Suppressing Th17 Cell Differentiation
- PMID: 26368822
- PMCID: PMC4569406
- DOI: 10.1371/journal.pone.0137711
Identification of Tetraazacyclic Compounds as Novel Potent Inhibitors Antagonizing RORγt Activity and Suppressing Th17 Cell Differentiation
Abstract
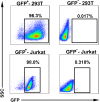
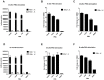
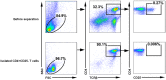
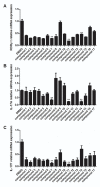
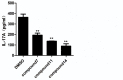
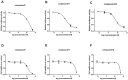
CD4+ T-helper cells that produce interleukin-17 (Th17 cells) are characterized as pathological T-helper cells in autoimmune diseases. Differentiation of human and mouse Th17 cells requires a key transcription regulator, retinoic acid receptor-related orphan receptor γt (RORγt), which is a potential therapeutic target for autoimmune diseases. To develop a therapeutic agent for Th17-mediated autoimmune diseases, we have established a high-throughput screening (HTS) assay for candidate screening, in which the luciferase activity in RORγt-LBD positive and negative Jurkat cells were analyzed to evaluate induction of RORγt activity by compounds. This technique was applied to screen a commercially-available drug-like chemical compound library (Enamine) which contains 20155 compounds. The screening identified 17 compounds that can inhibit RORγt function in the HTS screen system. Of these, three tetraazacyclic compounds can potently inhibit RORγt activity, and suppress Th17 differentiation and IL-17 production. These three candidate compounds could significantly attenuate the expression of the Il17a by 65%- 90%, and inhibit IL-17A secretion by 47%, 63%, and 74%, respectively. These compounds also exhibited a potent anti-RORγt activity, with EC50 values of 0.25 μM, 0.67 μM and 2.6 μM, respectively. Our data demonstrated the feasibility of targeting the RORγt to inhibit Th17 cell differentiation and function with these tetraazacyclic compounds, and the potential to improve the structure of these compounds for autoimmune diseases therapeutics.
Conflict of interest statement
Figures


References
-
- Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Progress in nucleic acid research and molecular biology. 2001;69:205–47. Epub 2001/09/12. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

