Retinoic Acid Activity in Undifferentiated Neural Progenitors Is Sufficient to Fulfill Its Role in Restricting Fgf8 Expression for Somitogenesis
- PMID: 26368825
- PMCID: PMC4569375
- DOI: 10.1371/journal.pone.0137894
Retinoic Acid Activity in Undifferentiated Neural Progenitors Is Sufficient to Fulfill Its Role in Restricting Fgf8 Expression for Somitogenesis
Abstract
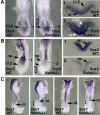
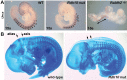
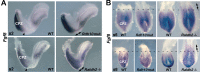
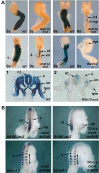
Bipotent axial stem cells residing in the caudal epiblast during late gastrulation generate neuroectodermal and presomitic mesodermal progeny that coordinate somitogenesis with neural tube formation, but the mechanism that controls these two fates is not fully understood. Retinoic acid (RA) restricts the anterior extent of caudal fibroblast growth factor 8 (Fgf8) expression in both mesoderm and neural plate to control somitogenesis and neurogenesis, however it remains unclear where RA acts to control the spatial expression of caudal Fgf8. Here, we found that mouse Raldh2-/- embryos, lacking RA synthesis and displaying a consistent small somite defect, exhibited abnormal expression of key markers of axial stem cell progeny, with decreased Sox2+ and Sox1+ neuroectodermal progeny and increased Tbx6+ presomitic mesodermal progeny. The Raldh2-/- small somite defect was rescued by treatment with an FGF receptor antagonist. Rdh10 mutants, with a less severe RA synthesis defect, were found to exhibit a small somite defect and anterior expansion of caudal Fgf8 expression only for somites 1-6, with normal somite size and Fgf8 expression thereafter. Rdh10 mutants were found to lack RA activity during the early phase when somites are small, but at the 6-somite stage RA activity was detected in neural plate although not in presomitic mesoderm. Expression of a dominant-negative RA receptor in mesoderm eliminated RA activity in presomitic mesoderm but did not affect somitogenesis. Thus, RA activity in the neural plate is sufficient to prevent anterior expansion of caudal Fgf8 expression associated with a small somite defect. Our studies provide evidence that RA restriction of Fgf8 expression in undifferentiated neural progenitors stimulates neurogenesis while also restricting the anterior extent of the mesodermal Fgf8 mRNA gradient that controls somite size, providing new insight into the mechanism that coordinates somitogenesis with neurogenesis.
Conflict of interest statement
Figures

References
-
- Kurlandsky SB, Gamble MV, Ramakrishnan R, Blaner WS (1995) Plasma delivery of retinoic acid to tissues in the rat. J Biol Chem 270: 17850–17857. - PubMed
-
- Horton C, Maden M (1995) Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev Dyn 202: 312–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

