RPE necroptosis in response to oxidative stress and in AMD
- PMID: 26369358
- PMCID: PMC4661094
- DOI: 10.1016/j.arr.2015.09.002
RPE necroptosis in response to oxidative stress and in AMD
Abstract
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the elderly. The underlying mechanism of non-neovascular AMD (dry AMD), also named geographic atrophy (GA) remains unclear and the mechanism of retinal pigment epithelial (RPE) cell death in AMD is controversial. We review the history and recent progress in understanding the mechanism of RPE cell death induced by oxidative stress, in AMD mouse models, and in AMD patients. Due to the limitation of toolsets to distinguish between apoptosis and necroptosis (or necrosis), most previous research concludes that apoptosis is a major mechanism for RPE cell death in response to oxidative stress and in AMD. Recent studies suggest necroptosis as a major mechanism of RPE cell death in response to oxidative stress. Moreover, ultrastructural and histopathological studies support necrosis as major mechanism of RPE cells death in AMD. In this review, we discuss the mechanism of RPE cell death in response to oxidative stress, in AMD mouse models, and in human AMD patients. Based on the literature, we hypothesize that necroptosis is a major mechanism for RPE cell death in response to oxidative stress and in AMD.
Keywords: Age-related macular degeneration; Apoptosis; Geographic atrophy; Necroptosis; Necrosis; Oxidative stress; RPE.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have no proprietary interest.
Figures
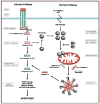
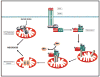
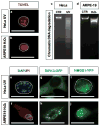

References
-
- Age-Related Eye Disease Study 2 Research G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. - PubMed
-
- Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of ophthalmology. 2001;119:1417–1436. - PMC - PubMed
-
- Alge CS, Priglinger SG, Neubauer AS, Kampik A, Zillig M, Bloemendal H, Welge-Lussen U. Retinal pigment epithelium is protected against apoptosis by alphaB-crystallin. Invest Ophthalmol Vis Sci. 2002;43:3575–3582. - PubMed
-
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. - PubMed
-
- Baich A, Ziegler M. The effect of sodium iodate and melanin on the formation of glyoxylate. Pigment cell research/sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 1992;5:394–395. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

