CYP2D6 genotype- and endoxifen-guided tamoxifen dose escalation increases endoxifen serum concentrations without increasing side effects
- PMID: 26369533
- PMCID: PMC4589558
- DOI: 10.1007/s10549-015-3562-5
CYP2D6 genotype- and endoxifen-guided tamoxifen dose escalation increases endoxifen serum concentrations without increasing side effects
Abstract
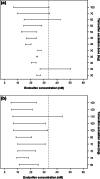
Breast cancer patients with absent or reduced CYP2D6 activity and consequently low endoxifen levels may benefit less from tamoxifen treatment. CYP2D6 poor and intermediate metabolizers may need a personalized increased tamoxifen dose to achieve effective endoxifen serum concentrations, without increasing toxicity. From a prospective study population of early breast cancer patients using tamoxifen (CYPTAM: NTR1509), 12 CYP2D6 poor and 12 intermediate metabolizers were selected and included in a one-step tamoxifen dose escalation study during 2 months. The escalated dose was calculated by multiplying the individual's endoxifen level at baseline relative to the average endoxifen concentration observed in CYP2D6 extensive metabolizers by 20 mg (120 mg maximum). Endoxifen levels and tamoxifen toxicity were determined at baseline and after 2 months, just before patients returned to the standard dose of 20 mg. Tamoxifen dose escalation in CYP2D6 poor and intermediate metabolizers significantly increased endoxifen concentrations (p < 0.001; p = 0.002, respectively) without increasing side effects. In intermediate metabolizers, dose escalation increased endoxifen to levels comparable with those observed in extensive metabolizers. In poor metabolizers, the mean endoxifen level increased from 24 to 81 % of the mean concentration in extensive metabolizers. In all patients, the endoxifen threshold of 5.97 ng/ml (=16.0 nM) reported by Madlensky et al. was reached following dose escalation. CYP2D6 genotype- and endoxifen-guided tamoxifen dose escalation increased endoxifen concentrations without increasing short-term side effects. Whether such tamoxifen dose escalation is effective and safe in view of long-term toxic effects is uncertain and needs to be explored.
Keywords: CYP2D6; Early breast cancer; Endoxifen.
Figures
References
-
- Schroth W, Hamann U, Fasching PA, Dauser S, Winter S, Eichelbaum M, et al. CYP2D6 polymorphisms as predictors of outcome in breast cancer patients treated with tamoxifen: expanded polymorphism coverage improves risk stratification. Clin Cancer Res. 2010;16:4468–4477. doi: 10.1158/1078-0432.CCR-10-0478. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

