Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy
- PMID: 26369565
- PMCID: PMC4593342
- DOI: 10.1186/s40880-015-0051-5
Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy
Abstract
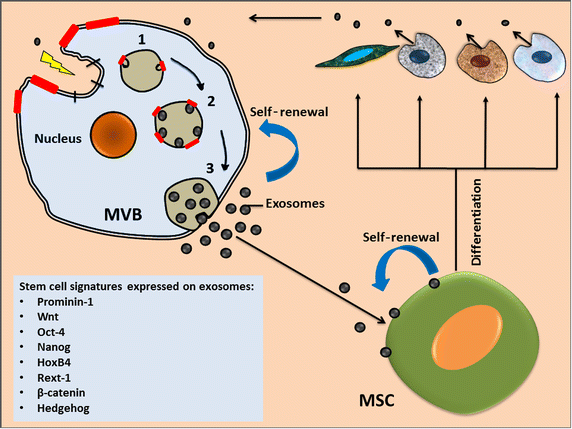
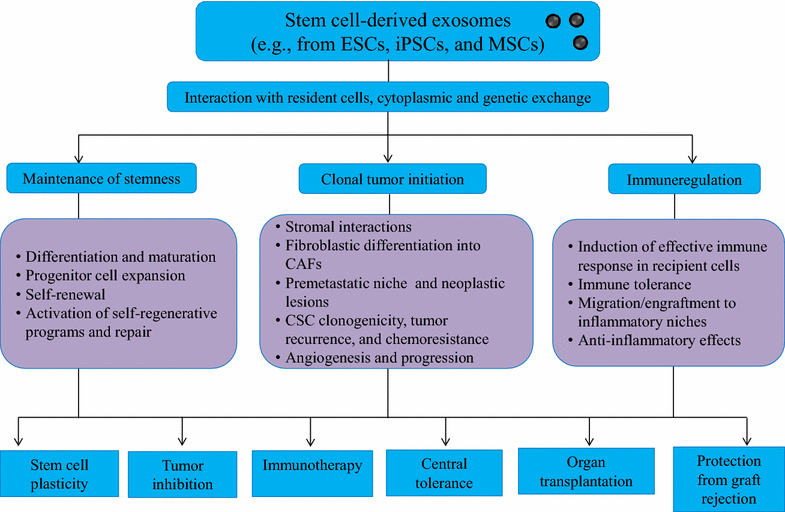
Stem cells are known to maintain stemness at least in part through secreted factors that promote stem-like phenotypes in resident cells. Accumulating evidence has clarified that stem cells release nano-vesicles, known as exosomes, which may serve as mediators of cell-to-cell communication and may potentially transmit stem cell phenotypes to recipient cells, facilitating stem cell maintenance, differentiation, self-renewal, and repair. It has become apparent that stem cell-derived exosomes mediate interactions among stromal elements, promote genetic instability in recipient cells, and induce malignant transformation. This review will therefore discuss the potential of stem cell-derived exosomes in the context of stromal remodeling and their ability to generate cancer-initiating cells in a tumor niche by inducing morphologic and functional differentiation of fibroblasts into tumor-initiating fibroblasts. In addition, the immunosuppressive potential of stem cell-derived exosomes in cancer immunotherapy and their prospective applications in cell-free therapies in future translational medicine is discussed.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

