Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry
- PMID: 26369815
- PMCID: PMC4877035
- DOI: 10.2174/1568026615666150915111741
Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry
Abstract
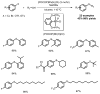
The impact of the development of sulfur therapeutics is instrumental to the evolution of the pharmaceutical industry. Sulfur-derived functional groups can be found in a broad range of pharmaceuticals and natural products. For centuries, sulfur continues to maintain its status as the dominating heteroatom integrated into a set of 362 sulfur-containing FDA approved drugs (besides oxygen or nitrogen) through the present. Sulfonamides, thioethers, sulfones and Penicillin are the most common scaffolds in sulfur containing drugs, which are well studied both on synthesis and application during the past decades. In this review, these four moieties in pharmaceuticals and recent advances in the synthesis of the corresponding core scaffolds are presented.
Conflict of interest statement
The authors confirm that this article content has no conflict of interest.
Figures

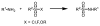

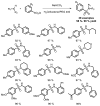
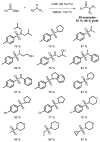

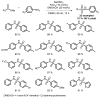

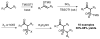






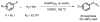













References
-
- Bernardi F, Csizmadia IG, Mangini A. In: Organic Sulfur Chemistry. Theoretical and Experimental Advances. Bernardi F, Csizmadia IG, Mangini A, editors. Elsevier; Amsterdam, The Netherlands: 1985.
- Block E. Reactions of Organosulfur Compounds. Academic Press; New York: 1978.
- Patai S, Rappoport Z. The Chemistry of Organic Selenium and Tellurium Compounds. John Wiley & Sons; New York: 1986–1987.
- McReynolds MD, Dougherty JM, Hanson PR. Synthesis of phosphorus and sulfur heterocycles via ring-closing olefin metathesis. Chem Rev. 2004;104:2239–2258. - PubMed
- Ager DJ. Silicon-containing carbonyl equivalents. Chem Soc Rev. 1982;11:493.
-
- Guo H, Sun B, Gao H, Chen X, Liu S, Yao X, Liu X, Che Y. Diketopiperazines from the cordyceps-colonizing fungus Epicoccum nigrum. J Nat Prod. 2009;72:2115–2119. - PubMed
- Wang J-M, Ding G-Z, Fang L, Dai J-G, Yu S-S, Wang Y-H, Chen X-G, Ma S-G, Qu J, Xu S, Du D. Thiodiketopiperazines produced by the endophytic fungus Epicoccum nigrum. J Nat Prod. 2010;73:1240–1249. - PubMed
-
- Thase ME, MacFadden W, Weisler RH, Chang W, Paulsson BR, Khan A, Calabrese JR, Bolder G. Efficacy of quetiapine monotherapy in bipolar I and II depression: A double-blind, placebo-controlled study (The BOLDER II Study) Journal of Clinical Psychopharmacology. 2006;26:600–609. - PubMed
-
- Lesch JE. The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine. Oxford University Press; 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
