Evaluation of VT-1161 for Treatment of Coccidioidomycosis in Murine Infection Models
- PMID: 26369964
- PMCID: PMC4649211
- DOI: 10.1128/AAC.00593-15
Evaluation of VT-1161 for Treatment of Coccidioidomycosis in Murine Infection Models
Abstract
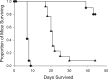
Coccidioidomycosis, or valley fever, is a growing health concern endemic to the southwestern United States. Safer, more effective, and more easily administered drugs are needed especially for severe, chronic, or unresponsive infections. The novel fungal CYP51 inhibitor VT-1161 demonstrated in vitro antifungal activity, with MIC50 and MIC90 values of 1 and 2 μg/ml, respectively, against 52 Coccidioides clinical isolates. In the initial animal study, oral doses of 10 and 50 mg/kg VT-1161 significantly reduced fungal burdens and increased survival time in a lethal respiratory model in comparison with treatment with a placebo (P < 0.001). Oral doses of 25 and 50 mg/kg VT-1161 were similarly efficacious in the murine central nervous system (CNS) model compared to placebo treatment (P < 0.001). All comparisons with the positive-control drug, fluconazole at 50 mg/kg per day, demonstrated either statistical equivalence or superiority of VT-1161. VT-1161 treatment also prevented dissemination of infection from the original inoculation site to a greater extent than fluconazole. Many of these in vivo results can be explained by the long half-life of VT-1161 leading to sustained high plasma levels. Thus, the efficacy and pharmacokinetics of VT-1161 are attractive characteristics for long-term treatment of this serious fungal infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

