Outcomes of Aminopenicillin Therapy for Vancomycin-Resistant Enterococcal Urinary Tract Infections
- PMID: 26369973
- PMCID: PMC4649236
- DOI: 10.1128/AAC.01817-15
Outcomes of Aminopenicillin Therapy for Vancomycin-Resistant Enterococcal Urinary Tract Infections
Abstract
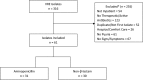
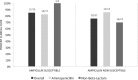
Vancomycin-resistant urinary tract infections are often challenging to treat. This retrospective cohort study compared outcomes between patients treated for vancomycin-resistant enterococcal urinary tract infection with an aminopenicillin and those treated with a non-β-lactam antibiotic. Inpatients treated with an enterococcus-active agent for their first symptomatic vancomycin-resistant enterococcal urinary tract infection between 1 January 2012 and 31 December 2013 were considered for inclusion. Patients with colonization, on hospice, or receiving comfort care only were excluded. The primary endpoint of clinical cure was defined as resolution of clinical symptoms, or symptom improvement to the extent that no additional antibacterial drug therapy was necessary, and lack of microbiologic persistence. Secondary endpoints of 30-day readmission or retreatment and 30-day all-cause mortality were also compared. A total of 316 urinary isolates were screened, and 61 patients with symptomatic urinary tract infection were included. Twenty (35%) of the 57 isolates tested were ampicillin susceptible. Thirty-one patients received an aminopenicillin, and 30 received a non-β-lactam. Rates of clinical cure for aminopenicillin versus non-β-lactam treatment were 26/31 (83.9%) and 22/30 (73.3%) (P = 0.315), respectively. Rates of 30-day readmission (6/31, or 19.4%, versus 9/30, or 30%, respectively; P = 0.334), 30-day retreatment (4/31, or 12.9%, versus 4/30, 13.3%, respectively; P = 0.960), and 30-day all-cause mortality (2/31, or 6.5%, versus 1/30, or 3.3%, respectively; P = 0.573) were also not significantly different between groups. Aminopenicillins may be a viable option for treating vancomycin-resistant urinary tract infection regardless of the organism's ampicillin susceptibility. Prospective validation with larger cohorts of patients should be considered.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Comment in
-
Urinary Tract Infections: Resistance Is Futile.Antimicrob Agents Chemother. 2016 Mar 25;60(4):2596-7. doi: 10.1128/AAC.00006-16. Print 2016 Apr. Antimicrob Agents Chemother. 2016. PMID: 27016557 Free PMC article. No abstract available.
-
Reply to "Urinary Tract Infections: Resistance Is Futile".Antimicrob Agents Chemother. 2016 Mar 25;60(4):2598. doi: 10.1128/AAC.00011-16. Print 2016 Apr. Antimicrob Agents Chemother. 2016. PMID: 27016558 Free PMC article. No abstract available.
References
-
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK, the National Healthcare Safety Network Team, Participating National Healthcare Safety Network Facilities. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 29:996–1011. doi:10.1086/591861. - DOI - PubMed
-
- Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. 2007. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis 58:163–170. doi:10.1016/j.diagmicrobio.2006.12.022. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical