Comparative genomic analyses of freshly isolated Giardia intestinalis assemblage A isolates
- PMID: 26370391
- PMCID: PMC4570179
- DOI: 10.1186/s12864-015-1893-6
Comparative genomic analyses of freshly isolated Giardia intestinalis assemblage A isolates
Abstract
Background: The diarrhea-causing protozoan Giardia intestinalis makes up a species complex of eight different assemblages (A-H), where assemblage A and B infect humans. Comparative whole-genome analyses of three of these assemblages have shown that there is significant divergence at the inter-assemblage level, however little is currently known regarding variation at the intra-assemblage level. We have performed whole genome sequencing of two sub-assemblage AII isolates, recently axenized from symptomatic human patients, to study the biological and genetic diversity within assemblage A isolates.
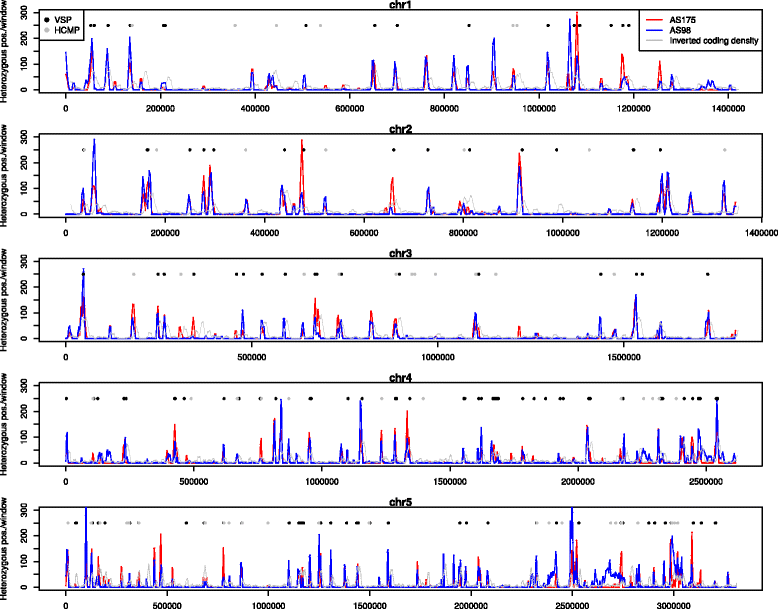
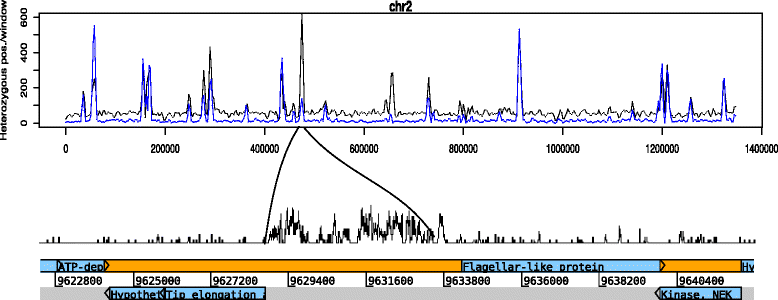
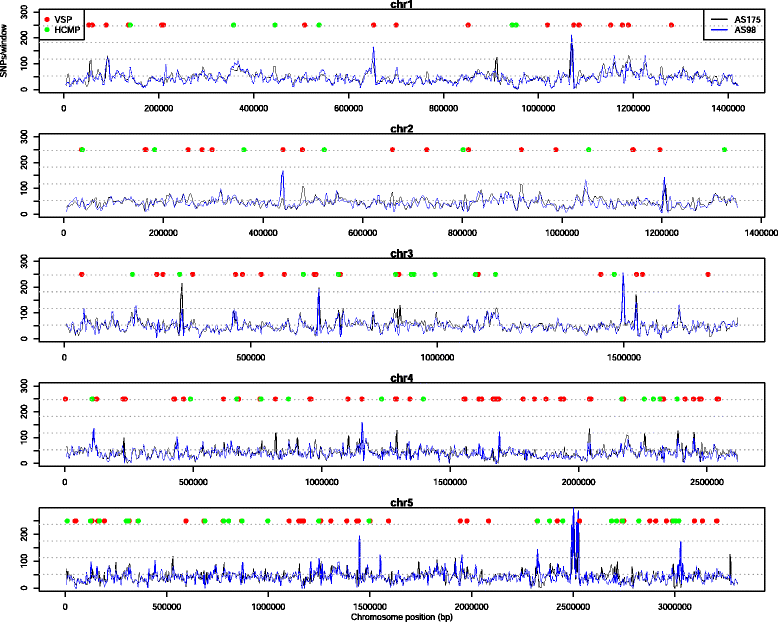
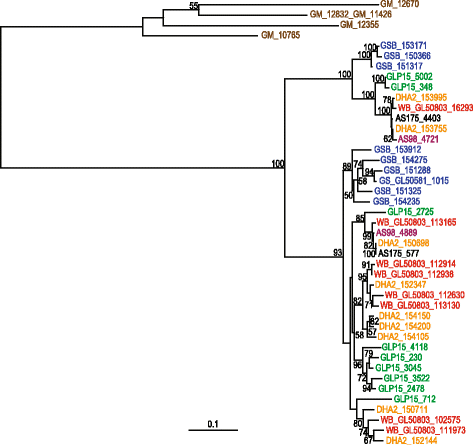
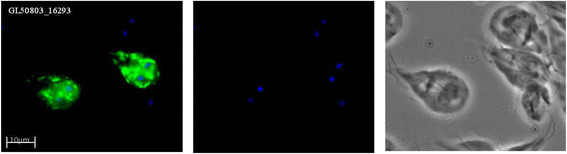
Results: Several biological differences between the new and earlier characterized assemblage A isolates were identified, including a difference in growth medium preference. The two AII isolates were of different sub-assemblage types (AII-1 [AS175] and AII-2 [AS98]) and showed size differences in the smallest chromosomes. The amount of genetic diversity was characterized in relation to the genome of the Giardia reference isolate WB, an assemblage AI isolate. Our analyses indicate that the divergence between AI and AII is approximately 1 %, represented by ~100,000 single nucleotide polymorphisms (SNP) distributed over the chromosomes with enrichment in variable genomic regions containing surface antigens. The level of allelic sequence heterozygosity (ASH) in the two AII isolates was found to be 0.25-0.35 %, which is 25-30 fold higher than in the WB isolate and 10 fold higher than the assemblage AII isolate DH (0.037 %). 35 protein-encoding genes, not found in the WB genome, were identified in the two AII genomes. The large gene families of variant-specific surface proteins (VSPs) and high cysteine membrane proteins (HCMPs) showed isolate-specific divergences of the gene repertoires. Certain genes, often in small gene families with 2 to 8 members, localize to the variable regions of the genomes and show high sequence diversity between the assemblage A isolates. One of the families, Bactericidal/Permeability Increasing-like protein (BPIL), with eight members was characterized further and the proteins were shown to localize to the ER in trophozoites.
Conclusions: Giardia genomes are modular with highly conserved core regions mixed up by variable regions containing high levels of ASH, SNPs and variable surface antigens. There are significant genomic variations in assemblage A isolates, in terms of chromosome size, gene content, surface protein repertoire and gene polymorphisms and these differences mainly localize to the variable regions of the genomes. The large genetic differences within one assemblage of G. intestinalis strengthen the argument that the assemblages represent different Giardia species.
Figures

References
-
- Ankarklev J, Jerlstrom-Hultqvist J, Ringqvist E, Troell K, Svard SG. Behind the smile: cell biology and disease mechanisms of Giardia species. Nat Rev Microbiol. 2010;8:413–422. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

