High CO2 Leads to Na,K-ATPase Endocytosis via c-Jun Amino-Terminal Kinase-Induced LMO7b Phosphorylation
- PMID: 26370512
- PMCID: PMC4628060
- DOI: 10.1128/MCB.00813-15
High CO2 Leads to Na,K-ATPase Endocytosis via c-Jun Amino-Terminal Kinase-Induced LMO7b Phosphorylation
Abstract
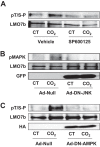
The c-Jun amino-terminal kinase (JNK) plays a role in inflammation, proliferation, apoptosis, and cell adhesion and cell migration by phosphorylating paxillin and β-catenin. JNK phosphorylation downstream of AMP-activated protein kinase (AMPK) activation is required for high CO2 (hypercapnia)-induced Na,K-ATPase endocytosis in alveolar epithelial cells. Here, we provide evidence that during hypercapnia, JNK promotes the phosphorylation of LMO7b, a scaffolding protein, in vitro and in intact cells. LMO7b phosphorylation was blocked by exposing the cells to the JNK inhibitor SP600125 and by infecting cells with dominant-negative JNK or AMPK adenovirus. The knockdown of the endogenous LMO7b or overexpression of mutated LMO7b with alanine substitutions of five potential JNK phosphorylation sites (LMO7b-5SA) or only Ser-1295 rescued both LMO7b phosphorylation and the hypercapnia-induced Na,K-ATPase endocytosis. Moreover, high CO2 promoted the colocalization and interaction of LMO7b and the Na,K-ATPase α1 subunit at the plasma membrane, which were prevented by SP600125 or by transfecting cells with LMO7b-5SA. Collectively, our data suggest that hypercapnia leads to JNK-induced LMO7b phosphorylation at Ser-1295, which facilitates the interaction of LMO7b with Na,K-ATPase at the plasma membrane promoting the endocytosis of Na,K-ATPase in alveolar epithelial cells.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






Similar articles
-
AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis.J Clin Invest. 2008 Feb;118(2):752-62. doi: 10.1172/JCI29723. J Clin Invest. 2008. PMID: 18188452 Free PMC article.
-
Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta.Mol Cell Biol. 2009 Jul;29(13):3455-64. doi: 10.1128/MCB.00054-09. Epub 2009 Apr 20. Mol Cell Biol. 2009. PMID: 19380482 Free PMC article.
-
Evolutionary conserved role of c-Jun-N-terminal kinase in CO2-induced epithelial dysfunction.PLoS One. 2012;7(10):e46696. doi: 10.1371/journal.pone.0046696. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056407 Free PMC article.
-
Regulation of Na,K-ATPase during acute lung injury.J Bioenerg Biomembr. 2007 Dec;39(5-6):391-5. doi: 10.1007/s10863-007-9102-1. J Bioenerg Biomembr. 2007. PMID: 17972021 Review.
-
Effects of hypercapnia on the lung.J Physiol. 2017 Apr 15;595(8):2431-2437. doi: 10.1113/JP273781. Epub 2017 Feb 14. J Physiol. 2017. PMID: 28044311 Free PMC article. Review.
Cited by
-
Carbon dioxide-dependent signal transduction in mammalian systems.Interface Focus. 2021 Apr 6;11(2):20200033. doi: 10.1098/rsfs.2020.0033. Epub 2021 Feb 12. Interface Focus. 2021. PMID: 33633832 Free PMC article. Review.
-
Sensing molecular carbon dioxide: a translational focus for respiratory disease.Physiol Rev. 2025 Oct 1;105(4):2657-2691. doi: 10.1152/physrev.00022.2024. Epub 2025 Jul 16. Physiol Rev. 2025. PMID: 40668657 Free PMC article. Review.
-
Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome.Intensive Care Med. 2017 Feb;43(2):200-208. doi: 10.1007/s00134-016-4611-1. Epub 2017 Jan 20. Intensive Care Med. 2017. PMID: 28108768 Free PMC article.
-
Optimizing Mechanical Ventilation Strategies in ARDS: The Role of Driving Pressure and Low Tidal Volume Ventilation.Crit Care Res Pract. 2025 Jul 26;2025:8857930. doi: 10.1155/ccrp/8857930. eCollection 2025. Crit Care Res Pract. 2025. PMID: 40756691 Free PMC article. Review.
-
A Non-Functional Carbon Dioxide-Mediated Post-Translational Modification on Nucleoside Diphosphate Kinase of Arabidopsis thaliana.Int J Mol Sci. 2024 Jan 11;25(2):898. doi: 10.3390/ijms25020898. Int J Mol Sci. 2024. PMID: 38255974 Free PMC article.
References
-
- Kohnlein T, Windisch W, Wegscheider K, Welte T. 2014. Non-invasive positive pressure ventilation for severe COPD. Authors' reply. Lancet Respir Med 2:e19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
