Development of a hematogenous implant-related infection in a rat model
- PMID: 26370721
- PMCID: PMC4570216
- DOI: 10.1186/s12891-015-0699-7
Development of a hematogenous implant-related infection in a rat model
Abstract
Background: Implant-related osteomyelitis is a major complication that requires immediate treatment, often involving removal of the implant, prolonging patient recovery and inflating expenses. Current research involving interventions to diminish the prevalence of such measures include investigating prophylactic and therapeutic remedies. A proper and accurate animal model is needed to thoroughly investigate such treatments. The scope of this project was to develop an animal model in which a consistent and measurable infection can be formed on an orthopedic implant when bacteria is introduced via a hematogenous source.
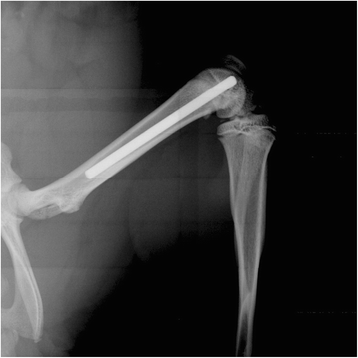
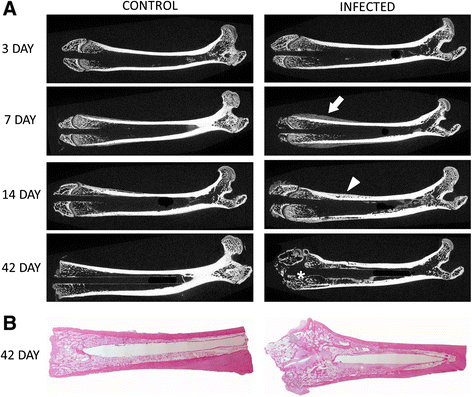
Methods: Titanium Kirschner-wires were implanted into the intramedullary canals of both femurs. Staphylococcus aureus, ranging from10(4) to 10(9) colony forming units, was injected into a tail vessel. After a designated time (3, 7, 14, or 42 days) the femurs were harvested and bacterial numbers determined for both the femur and the implanted K-wire. In addition, histology and micro-computed tomography were used as subjective tools to further characterize the infection.
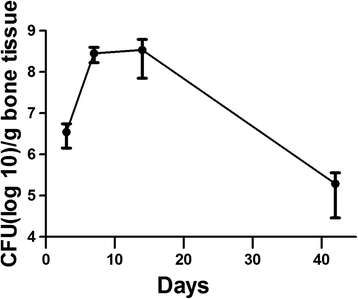
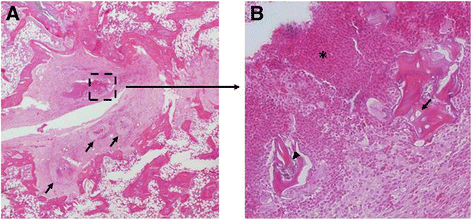
Results: Consistent infection, that is infection of ≥75% of the femurs, wasn't achieved until 10(7) CFU S. aureus was injected. At 10(7) CFU, the femurs contained 4.6x10(6) CFU/g bone tissue at day 3 and 4.8×10(8) CFU/g bone tissue by day 14. The wire showed comparable contamination with 4.8×10(4) CFU/mm(2) at day 3 and 3.7×10(5)/mm(2) by day 14. After 42 days, the bacteria number decreased but was still occupying at 1.9×10(5) CFU/g bone tissue. There were morphological changes to the bone as well. At day 42, there were signs of osteonecrosis and active bone formation when compared to control animals that received a K-wire but were inoculated with saline.
Conclusions: A model for hematogenous osteomyelitis, a common complication associated with implants, has been introduced. A reproducible, preclinical model is essential to evaluate future methods used to mitigate blood-borne bacteria hardware and bone infections.
Figures



References
-
- Johansen LK, Jensen HE. Animal models of hematogenous Staphylococcus aureus osteomyelitis in long bones: a review. Orthop. Res. Rev. 2013;5:51–64. doi: 10.2147/ORR.S45617. - DOI
-
- Horst SA, Hoerr V, Beineke A, Kreis C, Tuchscherr L, Kalinka J, Lehne S, Schleicher I, Kohler G, Fuchs T, et al. A novel mouse model of Staphylococcus aureus chronic osteomyelitis that closely mimics the human infection: an integrated view of disease pathogenesis. Am J Pathol. 2012;181(4):1206–14. doi: 10.1016/j.ajpath.2012.07.005. - DOI - PubMed
-
- Johansen LK, Frees D, Aalbaek B, Koch J, Iburg T, Nielsen OL, Leifsson PS, Jensen HE. A porcine model of acute, haematogenous, localized osteomyelitis due to Staphylococcus aureus: a pathomorphological study. Acta Pathol. Microbiol. Immunol. Scand. 2011;119(2):111–8. doi: 10.1111/j.1600-0463.2010.02700.x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

