Apremilast, a novel phosphodiesterase 4 (PDE4) inhibitor, regulates inflammation through multiple cAMP downstream effectors
- PMID: 26370839
- PMCID: PMC4570588
- DOI: 10.1186/s13075-015-0771-6
Apremilast, a novel phosphodiesterase 4 (PDE4) inhibitor, regulates inflammation through multiple cAMP downstream effectors
Abstract
Introduction: This work was undertaken to delineate intracellular signaling pathways for the PDE4 inhibitor apremilast and to examine interactions between apremilast, methotrexate and adenosine A2A receptors (A2AR).
Methods: After apremilast and LPS incubation, intracellular cAMP, TNF-α, IL-10, IL-6 and IL-1α were measured in the Raw264.7 monocytic murine cell line. PKA, Epac1/2 (signaling intermediates for cAMP) and A2AR knockdowns were performed by shRNA transfection and interactions with A2AR and A2BR, as well as with methotrexate were tested in vitro and in the murine air pouch model. Statistical differences were determined using one or two-way ANOVA or Student's t test. The alpha nominal level was set at 0.05 in all cases. A P value of < 0.05 was considered significant.
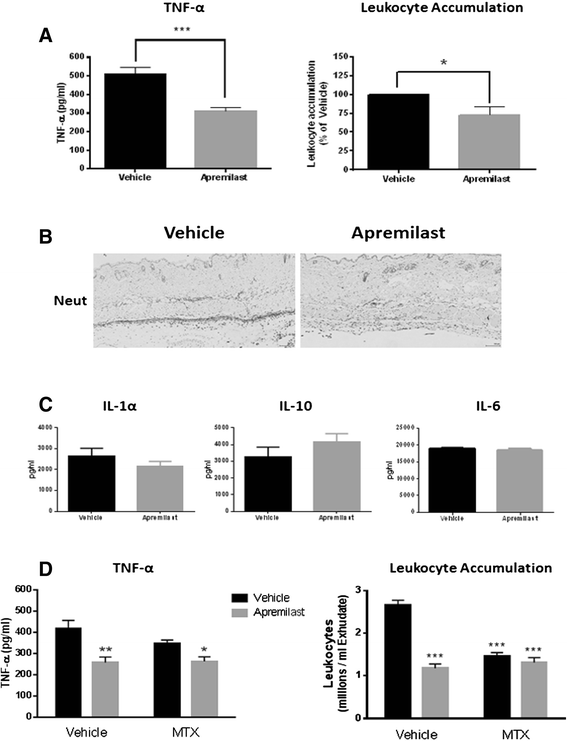
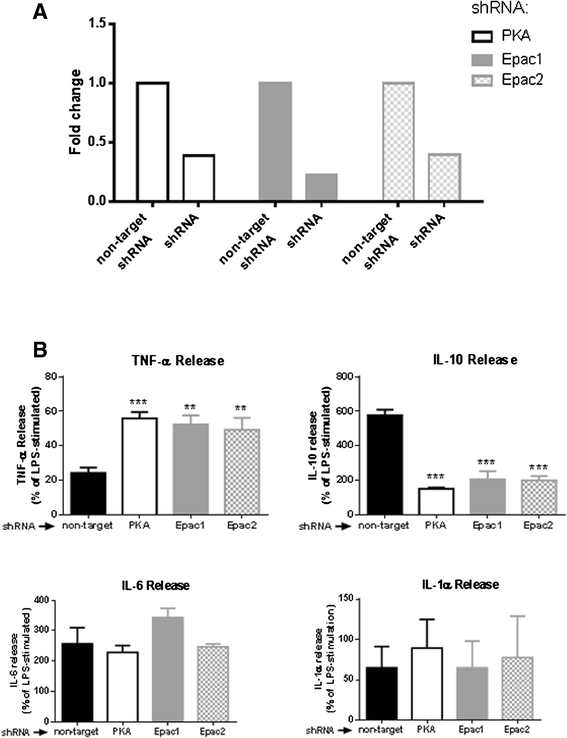
Results: In vitro, apremilast increased intracellular cAMP and inhibited TNF-α release (IC50=104nM) and the specific A2AR-agonist CGS21680 (1μM) increased apremilast potency (IC50=25nM). In this cell line, apremilast increased IL-10 production. PKA, Epac1 and Epac2 knockdowns prevented TNF-α inhibition and IL-10 stimulation by apremilast. In the murine air pouch model, both apremilast and MTX significantly inhibited leukocyte infiltration, while apremilast, but not MTX, significantly inhibited TNF-α release. The addition of MTX (1 mg/kg) to apremilast (5 mg/kg) yielded no more inhibition of leukocyte infiltration or TNF-α release than with apremilast alone.
Conclusions: The immunoregulatory effects of apremilast appear to be mediated by cAMP through the downstream effectors PKA, Epac1, and Epac2. A2AR agonism potentiated TNF-α inhibition by apremilast, consistent with the cAMP-elevating effects of that receptor. Because the A2AR is also involved in the anti-inflammatory effects of MTX, the mechanism of action of both drugs involves cAMP-dependent pathways and is therefore partially overlapping in nature.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

