Lipopolysaccharide transport to the cell surface: biosynthesis and extraction from the inner membrane
- PMID: 26370941
- PMCID: PMC4632605
- DOI: 10.1098/rstb.2015.0029
Lipopolysaccharide transport to the cell surface: biosynthesis and extraction from the inner membrane
Abstract
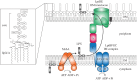
The cell surface of most Gram-negative bacteria is covered with lipopolysaccharide (LPS). The network of charges and sugars provided by the dense packing of LPS molecules in the outer leaflet of the outer membrane interferes with the entry of hydrophobic compounds into the cell, including many antibiotics. In addition, LPS can be recognized by the immune system and plays a crucial role in many interactions between bacteria and their animal hosts. LPS is synthesized in the inner membrane of Gram-negative bacteria, so it must be transported across their cell envelope to assemble at the cell surface. Over the past two decades, much of the research on LPS biogenesis has focused on the discovery and understanding of Lpt, a multi-protein complex that spans the cell envelope and functions to transport LPS from the inner membrane to the outer membrane. This paper focuses on the early steps of the transport of LPS by the Lpt machinery: the extraction of LPS from the inner membrane. The accompanying paper (May JM, Sherman DJ, Simpson BW, Ruiz N, Kahne D. 2015 Phil. Trans. R. Soc. B 370, 20150027. (doi:10.1098/rstb.2015.0027)) describes the subsequent steps as LPS travels through the periplasm and the outer membrane to its final destination at the cell surface.
Keywords: glycolipid; lpx; membrane biogenesis; permeability barrier.
© 2015 The Author(s).
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
