Toll-like receptor 9 signaling in dendritic cells regulates neutrophil recruitment to inflammatory foci following Leishmania infantum infection
- PMID: 26371124
- PMCID: PMC4645391
- DOI: 10.1128/IAI.00975-15
Toll-like receptor 9 signaling in dendritic cells regulates neutrophil recruitment to inflammatory foci following Leishmania infantum infection
Abstract
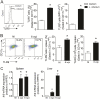
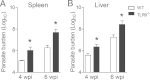
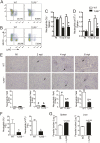
Leishmania infantum is a protozoan parasite that causes visceral leishmaniasis (VL). This infection triggers dendritic cell (DC) activation through the recognition of microbial products by Toll-like receptors (TLRs). Among the TLRs, TLR9 is required for DC activation by different Leishmania species. We demonstrated that TLR9 is upregulated in vitro and in vivo during infection. We show that C57BL/6 mice deficient in TLR9 expression (TLR9(-/-) mice) are more susceptible to infection and display higher parasite numbers in the spleen and liver. The increased susceptibility of TLR9(-/-) mice was due to the impaired recruitment of neutrophils to the infection foci associated with reduced levels of neutrophil chemoattractants released by DCs in the target organs. Moreover, both Th1 and Th17 cells were also committed in TLR9(-/-) mice. TLR9-dependent neutrophil recruitment is mediated via the MyD88 signaling pathway but is TIR domain-containing adapter-inducing interferon beta (TRIF) independent. Furthermore, L. infantum failed to activate both plasmacytoid and myeloid DCs from TLR9(-/-) mice, which presented reduced surface costimulatory molecule expression and chemokine release. Interestingly, neutrophil chemotaxis was affected both in vitro and in vivo when DCs were derived from TLR9(-/-) mice. Our results suggest that TLR9 plays a critical role in neutrophil recruitment during the protective response against L. infantum infection that could be associated with DC activation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


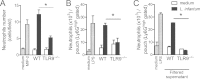

Similar articles
-
NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs.J Exp Med. 2007 Apr 16;204(4):893-906. doi: 10.1084/jem.20061293. Epub 2007 Mar 26. J Exp Med. 2007. PMID: 17389237 Free PMC article.
-
TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions.J Immunol. 2009 Feb 1;182(3):1386-96. doi: 10.4049/jimmunol.182.3.1386. J Immunol. 2009. PMID: 19155485
-
Interleukin-27 (IL-27) Mediates Susceptibility to Visceral Leishmaniasis by Suppressing the IL-17-Neutrophil Response.Infect Immun. 2016 Jul 21;84(8):2289-2298. doi: 10.1128/IAI.00283-16. Print 2016 Aug. Infect Immun. 2016. PMID: 27245409 Free PMC article.
-
Neutrophil recognition of bacterial DNA and Toll-like receptor 9-dependent and -independent regulation of neutrophil function.Arch Immunol Ther Exp (Warsz). 2008 Jan-Feb;56(1):41-53. doi: 10.1007/s00005-008-0008-3. Epub 2008 Feb 5. Arch Immunol Ther Exp (Warsz). 2008. PMID: 18250968 Review.
-
Balancing immunity and pathology in visceral leishmaniasis.Immunol Cell Biol. 2007 Feb-Mar;85(2):138-47. doi: 10.1038/sj.icb7100011. Epub 2006 Dec 5. Immunol Cell Biol. 2007. PMID: 17146466 Review.
Cited by
-
PRMT5 inhibition modulates murine dendritic cells activation by inhibiting the metabolism switch: a new therapeutic target in periodontitis.Ann Transl Med. 2021 May;9(9):755. doi: 10.21037/atm-20-7362. Ann Transl Med. 2021. PMID: 34268368 Free PMC article.
-
The role of TLR9 on Leishmania amazonensis infection and its influence on intranasal LaAg vaccine efficacy.PLoS Negl Trop Dis. 2019 Feb 25;13(2):e0007146. doi: 10.1371/journal.pntd.0007146. eCollection 2019 Feb. PLoS Negl Trop Dis. 2019. PMID: 30802247 Free PMC article.
-
The Impact of Neutrophil Recruitment to the Skin on the Pathology Induced by Leishmania Infection.Front Immunol. 2021 Mar 1;12:649348. doi: 10.3389/fimmu.2021.649348. eCollection 2021. Front Immunol. 2021. PMID: 33732265 Free PMC article. Review.
-
Leishmania infantum Parasites Subvert the Host Inflammatory Response through the Adenosine A2A Receptor to Promote the Establishment of Infection.Front Immunol. 2017 Jul 20;8:815. doi: 10.3389/fimmu.2017.00815. eCollection 2017. Front Immunol. 2017. PMID: 28775724 Free PMC article.
-
Toll-like Receptor-9 (TLR-9) Signaling Is Crucial for Inducing Protective Immunity following Immunization with Genetically Modified Live Attenuated Leishmania Parasites.Pathogens. 2023 Mar 29;12(4):534. doi: 10.3390/pathogens12040534. Pathogens. 2023. PMID: 37111420 Free PMC article.
References
-
- Debierre-Grockiego F, Azzouz N, Schmidt J, Dubremetz J-F, Geyer H, Geyer R, Weingart R, Schmidt RR, Schwarz RT. 2003. Roles of glycosylphosphatidylinositols of Toxoplasma gondii induction of tumor necrosis factor-α production in macrophages. J Biol Chem 278:32987–32993. doi:10.1074/jbc.M304791200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

