Pulmonary immunostimulation with MALP-2 in influenza virus-infected mice increases survival after pneumococcal superinfection
- PMID: 26371127
- PMCID: PMC4645412
- DOI: 10.1128/IAI.00948-15
Pulmonary immunostimulation with MALP-2 in influenza virus-infected mice increases survival after pneumococcal superinfection
Abstract
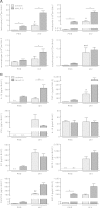
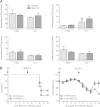
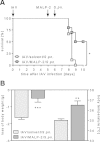
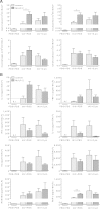
Pulmonary infection with influenza virus is frequently complicated by bacterial superinfection, with Streptococcus pneumoniae being the most prevalent causal pathogen and hence often associated with high morbidity and mortality rates. Local immunosuppression due to pulmonary influenza virus infection has been identified as a major cause of the pathogenesis of secondary bacterial lung infection. Thus, specific local stimulation of the pulmonary innate immune system in subjects with influenza virus infection might improve the host defense against secondary bacterial pathogens. In the present study, we examined the effect of pulmonary immunostimulation with Toll-like receptor 2 (TLR-2)-stimulating macrophage-activating lipopeptide 2 (MALP-2) in influenza A virus (IAV)-infected mice on the course of subsequent pneumococcal superinfection. Female C57BL/6N mice infected with IAV were treated with MALP-2 on day 5 and challenged with S. pneumoniae on day 6. Intratracheal MALP-2 application increased proinflammatory cytokine and chemokine release and enhanced the recruitment of leukocytes, mainly neutrophils, into the alveolar space of IAV-infected mice, without detectable systemic side effects. Local pulmonary instillation of MALP-2 in IAV-infected mice 24 h before transnasal pneumococcal infection considerably reduced the bacterial number in the lung tissue without inducing exaggerated inflammation. The pulmonary viral load was not altered by MALP-2. Clinically, MALP-2 treatment of IAV-infected mice increased survival rates and reduced hypothermia and body weight loss after pneumococcal superinfection compared to those of untreated coinfected mice. In conclusion, local immunostimulation with MALP-2 in influenza virus-infected mice improved pulmonary bacterial elimination and increased survival after subsequent pneumococcal superinfection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
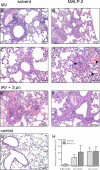
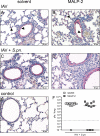

Similar articles
-
Immunostimulation with macrophage-activating lipopeptide-2 increased survival in murine pneumonia.Am J Respir Cell Mol Biol. 2009 Apr;40(4):474-81. doi: 10.1165/rcmb.2008-0071OC. Epub 2008 Oct 17. Am J Respir Cell Mol Biol. 2009. PMID: 18931326
-
The effect of intravenous peramivir, compared with oral oseltamivir, on the outcome of post-influenza pneumococcal pneumonia in mice.Antivir Ther. 2015;20(1):11-9. doi: 10.3851/IMP2744. Epub 2014 Feb 12. Antivir Ther. 2015. PMID: 24517996
-
Pneumococcal conjugate vaccine modulates macrophage-mediated innate immunity in pneumonia caused by Streptococcus pneumoniae following influenza.Microbes Infect. 2020 Sep;22(8):312-321. doi: 10.1016/j.micinf.2019.12.005. Epub 2020 Jan 17. Microbes Infect. 2020. PMID: 31958572
-
Inflammation as a Modulator of Host Susceptibility to Pulmonary Influenza, Pneumococcal, and Co-Infections.Front Immunol. 2020 Feb 11;11:105. doi: 10.3389/fimmu.2020.00105. eCollection 2020. Front Immunol. 2020. PMID: 32117259 Free PMC article. Review.
-
The Contribution of Viral Proteins to the Synergy of Influenza and Bacterial Co-Infection.Viruses. 2022 May 16;14(5):1064. doi: 10.3390/v14051064. Viruses. 2022. PMID: 35632805 Free PMC article. Review.
Cited by
-
IL-37 Causes Excessive Inflammation and Tissue Damage in Murine Pneumococcal Pneumonia.J Innate Immun. 2017;9(4):403-418. doi: 10.1159/000469661. Epub 2017 Jun 10. J Innate Immun. 2017. PMID: 28601872 Free PMC article.
-
Peptidoglycan from Immunobiotic Lactobacillus rhamnosus Improves Resistance of Infant Mice to Respiratory Syncytial Viral Infection and Secondary Pneumococcal Pneumonia.Front Immunol. 2017 Aug 10;8:948. doi: 10.3389/fimmu.2017.00948. eCollection 2017. Front Immunol. 2017. PMID: 28848552 Free PMC article.
-
Age-Dependent Progression of SARS-CoV-2 Infection in Syrian Hamsters.Viruses. 2020 Jul 20;12(7):779. doi: 10.3390/v12070779. Viruses. 2020. PMID: 32698441 Free PMC article.
-
Streptococcus pneumoniae PepO promotes host anti-infection defense via autophagy in a Toll-like receptor 2/4 dependent manner.Virulence. 2020 Dec;11(1):270-282. doi: 10.1080/21505594.2020.1739411. Virulence. 2020. PMID: 32172666 Free PMC article.
-
TLR Agonists as Mediators of Trained Immunity: Mechanistic Insight and Immunotherapeutic Potential to Combat Infection.Front Immunol. 2021 Feb 18;11:622614. doi: 10.3389/fimmu.2020.622614. eCollection 2020. Front Immunol. 2021. PMID: 33679711 Free PMC article. Review.
References
-
- Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk H-D, Meisel A. 2003. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med 198:725–736. doi:10.1084/jem.20021098. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

