Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function
- PMID: 26371171
- PMCID: PMC4666982
- DOI: 10.1152/ajpheart.00557.2015
Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function
Abstract
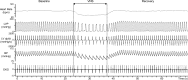
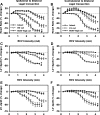
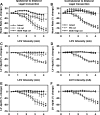
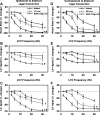
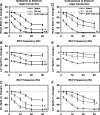
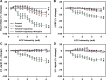
Using vagus nerve stimulation (VNS), we sought to determine the contribution of vagal afferents to efferent control of cardiac function. In anesthetized dogs, the right and left cervical vagosympathetic trunks were stimulated in the intact state, following ipsilateral or contralateral vagus nerve transection (VNTx), and then following bilateral VNTx. Stimulations were performed at currents from 0.25 to 4.0 mA, frequencies from 2 to 30 Hz, and a 500-μs pulse width. Right or left VNS evoked significantly greater current- and frequency-dependent suppression of chronotropic, inotropic, and lusitropic function subsequent to sequential VNTx. Bradycardia threshold was defined as the current first required for a 5% decrease in heart rate. The threshold for the right vs. left vagus-induced bradycardia in the intact state (2.91 ± 0.18 and 3.47 ± 0.20 mA, respectively) decreased significantly with right VNTx (1.69 ± 0.17 mA for right and 3.04 ± 0.27 mA for left) and decreased further following bilateral VNTx (1.29 ± 0.16 mA for right and 1.74 ± 0.19 mA for left). Similar effects were observed following left VNTx. The thresholds for afferent-mediated effects on cardiac parameters were 0.62 ± 0.04 and 0.65 ± 0.06 mA with right and left VNS, respectively, and were reflected primarily as augmentation. Afferent-mediated tachycardias were maintained following β-blockade but were eliminated by VNTx. The increased effectiveness and decrease in bradycardia threshold with sequential VNTx suggest that 1) vagal afferents inhibit centrally mediated parasympathetic efferent outflow and 2) the ipsilateral and contralateral vagi exert a substantial buffering capacity. The intact threshold reflects the interaction between multiple levels of the cardiac neural hierarchy.
Keywords: afferent; autonomic nervous system; intrinsic cardiac nervous system; parasympathetic; vagus nerve stimulation.
Copyright © 2015 the American Physiological Society.
Figures




References
-
- Andresen MC, Kunze DL, Mendelowitz D. Central nervous system regulation of the heart. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 187–219.
-
- Ardell JL, Beaumont E, Nier H, Southerland EM, Griffin S, KenKnight BH, Armour JA. Vagal nerve stimulation elicits heart rate responses that are dependent on relative activation of both central and peripheral limbs of the autonomic nervous system: defining the neural fulcrum (Abstract). Heart Rhythm. 2014.
-
- Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am J Physiol Heart Circ Physiol 260: H713–H721, 1991. - PubMed
-
- Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol Heart Circ Physiol 251: H764–H773, 1986. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

