Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott-Aldrich syndrome
- PMID: 26371186
- PMCID: PMC4577851
- DOI: 10.1084/jem.20150585
Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott-Aldrich syndrome
Abstract
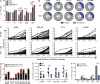
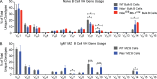
Wiskott-Aldrich syndrome (WAS) is an X-linked immunodeficiency disorder frequently associated with systemic autoimmunity, including autoantibody-mediated cytopenias. WAS protein (WASp)-deficient B cells have increased B cell receptor (BCR) and Toll-like receptor (TLR) signaling, suggesting that these pathways might impact establishment of the mature, naive BCR repertoire. To directly investigate this possibility, we evaluated naive B cell specificity and composition in WASp-deficient mice and WAS subjects (n = 12). High-throughput sequencing and single-cell cloning analysis of the BCR repertoire revealed altered heavy chain usage and enrichment for low-affinity self-reactive specificities in murine marginal zone and human naive B cells. Although negative selection mechanisms including deletion, anergy, and receptor editing were relatively unperturbed, WASp-deficient transitional B cells showed enhanced proliferation in vivo mediated by antigen- and Myd88-dependent signals. Finally, using both BCR sequencing and cell surface analysis with a monoclonal antibody recognizing an intrinsically autoreactive heavy chain, we show enrichment in self-reactive cells specifically at the transitional to naive mature B cell stage in WAS subjects. Our combined data support a model wherein modest alterations in B cell-intrinsic, BCR, and TLR signals in WAS, and likely other autoimmune disorders, are sufficient to alter B cell tolerance via positive selection of self-reactive transitional B cells.
© 2015 Kolhatkar et al.
Figures





References
-
- Becker-Herman S., Meyer-Bahlburg A., Schwartz M.A., Jackson S.W., Hudkins K.L., Liu C., Sather B.D., Khim S., Liggitt D., Song W., et al. . 2011. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J. Exp. Med. 208:2033–2042. 10.1084/jem.20110200 - DOI - PMC - PubMed
-
- Brigido M.M., Polymenis M., and Stollar B.D.. 1993. Role of mouse VH10 and VL gene segments in the specific binding of antibody to Z-DNA, analyzed with recombinant single chain Fv molecules. J. Immunol. 150:469–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI071163/AI/NIAID NIH HHS/United States
- R37 AI049660/AI/NIAID NIH HHS/United States
- R01 HL075453/HL/NHLBI NIH HHS/United States
- R01AI071163/AI/NIAID NIH HHS/United States
- R01AI084457/AI/NIAID NIH HHS/United States
- R01 AI084457/AI/NIAID NIH HHS/United States
- DP3DK097672/DK/NIDDK NIH HHS/United States
- P01 HL059561/HL/NHLBI NIH HHS/United States
- T32 GM007266/GM/NIGMS NIH HHS/United States
- 5P01HL059561/HL/NHLBI NIH HHS/United States
- U19 AI110483/AI/NIAID NIH HHS/United States
- DP3 DK097672/DK/NIDDK NIH HHS/United States
- R01HL075453/HL/NHLBI NIH HHS/United States
- U01 DK070430/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

