Acute-Phase Deaths from Murine Polymicrobial Sepsis Are Characterized by Innate Immune Suppression Rather Than Exhaustion
- PMID: 26371253
- PMCID: PMC4592823
- DOI: 10.4049/jimmunol.1500874
Acute-Phase Deaths from Murine Polymicrobial Sepsis Are Characterized by Innate Immune Suppression Rather Than Exhaustion
Abstract
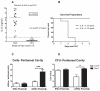
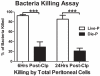
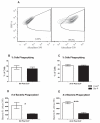
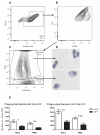
Sepsis, a leading cause of death in the United States, has poorly understood mechanisms of mortality. To address this, our model of cecal ligation and puncture (CLP) induced sepsis stratifies mice as predicted to Live (Live-P) or Die (Die-P) based on plasma IL-6. Six hours post-CLP, both Live-P and Die-P groups have equivalent peritoneal bacterial colony forming units and recruitment of phagocytes. By 24 h, however, Die-P mice have increased bacterial burden, despite increased neutrophil recruitment, suggesting Die-P phagocytes have impaired bacterial killing. Peritoneal cells were used to study multiple bactericidal processes: bacterial killing, reactive oxygen species (ROS) generation, and phagocytosis. Total phagocytosis and intraphagosomal processes were determined with triple-labeled Escherichia coli, covalently labeled with ROS- and pH-sensitive probes, and an ROS/pH-insensitive probe for normalization. Although similar proportions of Live-P and Die-P phagocytes responded to exogenous stimuli, Die-P phagocytes showed marked deficits in all parameters measured, thus suggesting immunosuppression rather than exhaustion. This contradicts the prevailing sepsis paradigm that acute-phase sepsis deaths (<5 d) result from excessive inflammation, whereas chronic-phase deaths (>5 d) are characterized by insufficient inflammation and immunosuppression. These data suggest that suppression of cellular innate immunity in sepsis occurs within the first 6 h.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures

References
-
- Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Critical care medicine. 2012;40:754–761. - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. The New England journal of medicine. 2003;348:1546–1554. - PubMed
-
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. - PubMed
-
- Torgersen C, Moser P, Luckner G, Mayr V, Jochberger S, Hasibeder WR, Dunser MW. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesthesia and analgesia. 2009;108:1841–1847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

