A Distinctive Cytoplasmic Tail Contributes to Low Surface Expression and Intracellular Retention of the Patr-AL MHC Class I Molecule
- PMID: 26371256
- PMCID: PMC4592827
- DOI: 10.4049/jimmunol.1500397
A Distinctive Cytoplasmic Tail Contributes to Low Surface Expression and Intracellular Retention of the Patr-AL MHC Class I Molecule
Abstract
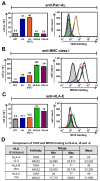
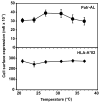
Chimpanzees have orthologs of the six fixed, functional human MHC class I genes. But, in addition, the chimpanzee has a seventh functional gene, Patr-AL, which is not polymorphic but contributes substantially to population diversity by its presence on only 50% of MHC haplotypes. The ancestral AL gene emerged long before the separation of human and chimpanzee ancestors and then subsequently and specifically lost function during human evolution, but was maintained in chimpanzees. Patr-AL is an alloantigen that participates in negative and positive selection of the T cell repertoire. The three-dimensional structure and the peptide-binding repertoire of Patr-AL and HLA-A*02 are surprisingly similar. In contrast, the expression of these two molecules is very different, as shown using specific mAbs and polyclonal Abs made against Patr-AL. Peripheral blood cells and B cell lines express low levels of Patr-AL at the cell surface. Higher levels are seen for 221-cell transfectants expressing Patr-AL, but in these cells a large majority of Patr-AL molecules are retained in the early compartments of the secretory pathway: mainly the endoplasmic reticulum, but also cis-Golgi. Replacing the cytoplasmic tail of Patr-AL with that of HLA-A*02 increased the cell-surface expression of Patr-AL substantially. Four substitutions distinguish the Patr-AL and HLA-A*02 cytoplasmic tails. Systematic mutagenesis showed that each substitution contributes changes in cell-surface expression. The combination of residues present in Patr-AL appears unique, but each individual residue is present in other primate MHC class I molecules, notably MHC-E, the most ancient of the functional human MHC class I molecules.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures







References
-
- Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. - PubMed
-
- Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–223. - PubMed
-
- Yang Y, Chu W, Geraghty DE, Hunt JS. Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-gamma. J Immunol. 1996;156:4224–4231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

