Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation
- PMID: 26371321
- PMCID: PMC4593117
- DOI: 10.1073/pnas.1509158112
Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation
Abstract
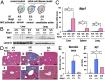
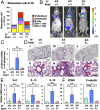
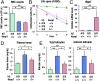
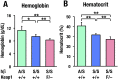
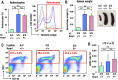
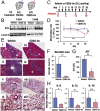
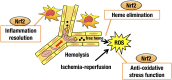
Sickle cell disease (SCD) is an inherited disorder caused by a point mutation in the β-globin gene, leading to the production of abnormally shaped red blood cells. Sickle cells are prone to hemolysis and thereby release free heme into plasma, causing oxidative stress and inflammation that in turn result in damage to multiple organs. The transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) is a master regulator of the antioxidant cell-defense system. Here we show that constitutive Nrf2 activation by ablation of its negative regulator Keap1 (kelch-like ECH-associated protein 1) significantly improves symptoms in SCD model mice. SCD mice exhibit severe liver damage and lung inflammation associated with high expression levels of proinflammatory cytokines and adhesion molecules compared with normal mice. Importantly, these symptoms subsided after Nrf2 activation. Although hemolysis and stress erythropoiesis did not change substantially in the Nrf2-activated SCD mice, Nrf2 promoted the elimination of plasma heme released by sickle cells' hemolysis and thereby reduced oxidative stress and inflammation, demonstrating that Nrf2 activation reduces organ damage and segregates inflammation from prevention of hemolysis in SCD mice. Furthermore, administration of the Nrf2 inducer CDDO-Im (2-cyano-3, 12 dioxooleana-1, 9 diene-28-imidazolide) also relieved inflammation and organ failure in SCD mice. These results support the contention that Nrf2 induction may be an important means to protect organs from the pathophysiology of sickle cell-induced damage.
Keywords: Keap1; Nrf2; sickle cell disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Dietary supplementation with sulforaphane attenuates liver damage and heme overload in a sickle cell disease murine model.Exp Hematol. 2019 Sep;77:51-60.e1. doi: 10.1016/j.exphem.2019.08.001. Epub 2019 Aug 9. Exp Hematol. 2019. PMID: 31404577
-
Control of Oxidative Stress and Inflammation in Sickle Cell Disease with the Nrf2 Activator Dimethyl Fumarate.Antioxid Redox Signal. 2017 May 10;26(14):748-762. doi: 10.1089/ars.2015.6571. Epub 2016 Mar 30. Antioxid Redox Signal. 2017. PMID: 26914345 Free PMC article.
-
Nrf2 protects against furosemide-induced hepatotoxicity.Toxicology. 2014 Oct 3;324:35-42. doi: 10.1016/j.tox.2014.02.008. Epub 2014 May 6. Toxicology. 2014. PMID: 24813929
-
Nrf2-Keap1 defines a physiologically important stress response mechanism.Trends Mol Med. 2004 Nov;10(11):549-57. doi: 10.1016/j.molmed.2004.09.003. Trends Mol Med. 2004. PMID: 15519281 Review.
-
Mechanisms of NRF2 activation to mediate fetal hemoglobin induction and protection against oxidative stress in sickle cell disease.Exp Biol Med (Maywood). 2019 Feb;244(2):171-182. doi: 10.1177/1535370219825859. Epub 2019 Jan 23. Exp Biol Med (Maywood). 2019. PMID: 30674214 Free PMC article. Review.
Cited by
-
Simvastatin-Mediated Nrf2 Activation Induces Fetal Hemoglobin and Antioxidant Enzyme Expression to Ameliorate the Phenotype of Sickle Cell Disease.Antioxidants (Basel). 2024 Mar 11;13(3):337. doi: 10.3390/antiox13030337. Antioxidants (Basel). 2024. PMID: 38539870 Free PMC article.
-
Flurbiprofen inhibits heme induced NLRP3 inflammasome in Berkeley sickle cell disease mice.Front Pharmacol. 2023 Apr 26;14:1123734. doi: 10.3389/fphar.2023.1123734. eCollection 2023. Front Pharmacol. 2023. PMID: 37180702 Free PMC article.
-
Reduced GATA1 levels are associated with ineffective erythropoiesis in sickle cell anemia.Haematologica. 2025 May 1;110(5):1150-1163. doi: 10.3324/haematol.2024.286010. Epub 2024 Dec 5. Haematologica. 2025. PMID: 39633531 Free PMC article.
-
MFSD7C protects hemolysis-induced lung impairments by inhibiting ferroptosis.Nat Commun. 2024 Sep 19;15(1):8226. doi: 10.1038/s41467-024-52537-6. Nat Commun. 2024. PMID: 39300060 Free PMC article.
-
Circulating mitochondrial DNA is a proinflammatory DAMP in sickle cell disease.Blood. 2021 Jun 3;137(22):3116-3126. doi: 10.1182/blood.2020009063. Blood. 2021. PMID: 33661274 Free PMC article. Clinical Trial.
References
-
- Ingram VM. Gene mutations in human haemoglobin: The chemical difference between normal and sickle cell haemoglobin. Nature. 1957;180(4581):326–328. - PubMed
-
- Li C, Wright MM, Jackson RM. Reactive species mediated injury of human lung epithelial cells after hypoxia-reoxygenation. Exp Lung Res. 2002;28(5):373–389. - PubMed
-
- Repka T, Hebbel RP. Hydroxyl radical formation by sickle erythrocyte membranes: Role of pathologic iron deposits and cytoplasmic reducing agents. Blood. 1991;78(10):2753–2758. - PubMed
-
- Akohoue SA, et al. Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediatr Res. 2007;61(2):233–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

