Clinicopathologic Characteristics of Microsatellite Instable Gastric Carcinomas Revisited: Urgent Need for Standardization
- PMID: 26371427
- PMCID: PMC5147042
- DOI: 10.1097/PAI.0000000000000264
Clinicopathologic Characteristics of Microsatellite Instable Gastric Carcinomas Revisited: Urgent Need for Standardization
Abstract
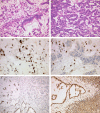
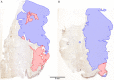
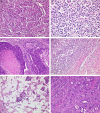
Microsatellite instable gastric cancer (MSI-GC) is a specific molecular subtype of GC. We studied the phenotypes, genotypes, and clinicopathologic characteristics of MSI-GC in a white GC cohort and compared our findings with an extended literature review. The study cohort consisted of 482 patients. Specimens were available from 452 cases and were used for immunostaining (MLH1, PMS2, MSH2, MSH6) and molecular biological analyses (BAT-25, BAT-26, NR-21, NR-24, NR-27; Epstein-Barr virus in situ hybridization). Thirty-four (7.5%) GCs were MSI. Loss of MLH1 and/or PMS2 was found in 30 (88%) MSI-GC, 3 (9%) showed loss of MSH2 and/or MSH6. One (3%) MSI-GC was identified only by molecular biological testing. A single case was heterogeneous and contained microsatellite-stable and instable tumor areas. Twenty-one (62%) MSI-GCs showed unusual histologic features. MSI-GC was not found in diffuse-type or Epstein-Barr virus-positive GC. MSI-GC was significantly more prevalent in elderly patients, distal stomach, and was associated with a significantly lower number of lymph node metastases and a significantly better overall and tumor-specific survival. MSI-GC constitutes a small but relevant subgroup of GC with distinct clinicopathologic characteristics. Our literature review illustrates the shortcomings of missing standardized testing algorithms with prevalences of MSI-GC ranging from 0% to 44.5%. Future studies should test the hypothesis that patients with MSI-GCs may not need adjuvant/perioperative chemotherapy. However, this will require a standardized, quality-controlled diagnostic algorithm of MSI for GC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. - PubMed
-
- Lauwers GY, Carneiro F, Graham DY, et al. Bosman FT, Carneiro F, Hruban RH, Theise ND. Tumours of the stomach. WHO Classification of Tumours of the Digestive System, 4th ed Lyon: International Agency for Research on Cancer; 2010:48–80.
-
- Lauren T. The two histologic main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

