siRNA Versus miRNA as Therapeutics for Gene Silencing
- PMID: 26372022
- PMCID: PMC4877448
- DOI: 10.1038/mtna.2015.23
siRNA Versus miRNA as Therapeutics for Gene Silencing
Abstract
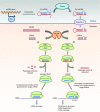
Discovered a little over two decades ago, small interfering RNAs (siRNAs) and microRNAs (miRNAs) are noncoding RNAs with important roles in gene regulation. They have recently been investigated as novel classes of therapeutic agents for the treatment of a wide range of disorders including cancers and infections. Clinical trials of siRNA- and miRNA-based drugs have already been initiated. siRNAs and miRNAs share many similarities, both are short duplex RNA molecules that exert gene silencing effects at the post-transcriptional level by targeting messenger RNA (mRNA), yet their mechanisms of action and clinical applications are distinct. The major difference between siRNAs and miRNAs is that the former are highly specific with only one mRNA target, whereas the latter have multiple targets. The therapeutic approaches of siRNAs and miRNAs are therefore very different. Hence, this review provides a comparison between therapeutic siRNAs and miRNAs in terms of their mechanisms of action, physicochemical properties, delivery, and clinical applications. Moreover, the challenges in developing both classes of RNA as therapeutics are also discussed.
Figures


Similar articles
-
Novel approaches in cancer treatment: preclinical and clinical development of small non-coding RNA therapeutics.J Exp Clin Cancer Res. 2021 Dec 4;40(1):383. doi: 10.1186/s13046-021-02193-1. J Exp Clin Cancer Res. 2021. PMID: 34863235 Free PMC article. Review.
-
Artificial miRNAs as therapeutic tools: Challenges and opportunities.Wiley Interdiscip Rev RNA. 2021 Jul;12(4):e1640. doi: 10.1002/wrna.1640. Epub 2021 Jan 1. Wiley Interdiscip Rev RNA. 2021. PMID: 33386705 Review.
-
Identification and characterization of microRNAs and endogenous siRNAs in Schistosoma japonicum.BMC Genomics. 2010 Jan 21;11:55. doi: 10.1186/1471-2164-11-55. BMC Genomics. 2010. PMID: 20092619 Free PMC article.
-
The Complexity of Posttranscriptional Small RNA Regulatory Networks Revealed by In Silico Analysis of Gossypium arboreum L. Leaf, Flower and Boll Small Regulatory RNAs.PLoS One. 2015 Jun 12;10(6):e0127468. doi: 10.1371/journal.pone.0127468. eCollection 2015. PLoS One. 2015. PMID: 26070200 Free PMC article.
-
RNAi Therapeutic Platforms for Lung Diseases.Pharmaceuticals (Basel). 2013 Feb 6;6(2):223-50. doi: 10.3390/ph6020223. Pharmaceuticals (Basel). 2013. PMID: 24275949 Free PMC article.
Cited by
-
Gene therapy with caspase-3 small interfering RNA-nanoparticles is neuroprotective after optic nerve damage.Neural Regen Res. 2021 Dec;16(12):2534-2541. doi: 10.4103/1673-5374.313068. Neural Regen Res. 2021. PMID: 33907045 Free PMC article.
-
Surface Design Options in Polymer- and Lipid-Based siRNA Nanoparticles Using Antibodies.Int J Mol Sci. 2022 Nov 11;23(22):13929. doi: 10.3390/ijms232213929. Int J Mol Sci. 2022. PMID: 36430411 Free PMC article. Review.
-
Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages.Cell Commun Signal. 2020 Sep 11;18(1):149. doi: 10.1186/s12964-020-00650-6. Cell Commun Signal. 2020. PMID: 32917227 Free PMC article. Review.
-
miR-142: A Master Regulator in Hematological Malignancies and Therapeutic Opportunities.Cells. 2023 Dec 30;13(1):84. doi: 10.3390/cells13010084. Cells. 2023. PMID: 38201290 Free PMC article. Review.
-
Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae.BMC Biotechnol. 2016 Jul 26;16(1):57. doi: 10.1186/s12896-016-0287-4. BMC Biotechnol. 2016. PMID: 27455996 Free PMC article.
References
-
- Mattick, JS and Makunin, IV (2006). Non-coding RNA. Hum Mol Genet 15 Spec No 1: R17–R29. - PubMed
-
- Tabernero, J, Shapiro, GI, LoRusso, PM, Cervantes, A, Schwartz, GK, Weiss, GJ et al. (2013). First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov 3: 406–417. - PubMed
-
- Schultheis, B, Strumberg, D, Santel, A, Vank, C, Gebhardt, F, Keil, O et al. (2014). First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol 32: 4141–4148. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

