Genome-Wide Scan and Test of Candidate Genes in the Snail Biomphalaria glabrata Reveal New Locus Influencing Resistance to Schistosoma mansoni
- PMID: 26372103
- PMCID: PMC4570800
- DOI: 10.1371/journal.pntd.0004077
Genome-Wide Scan and Test of Candidate Genes in the Snail Biomphalaria glabrata Reveal New Locus Influencing Resistance to Schistosoma mansoni
Abstract
Background: New strategies to combat the global scourge of schistosomiasis may be revealed by increased understanding of the mechanisms by which the obligate snail host can resist the schistosome parasite. However, few molecular markers linked to resistance have been identified and characterized in snails.
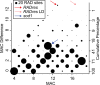
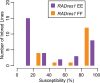
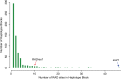
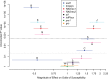
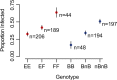
Methodology/principal findings: Here we test six independent genetic loci for their influence on resistance to Schistosoma mansoni strain PR1 in the 13-16-R1 strain of the snail Biomphalaria glabrata. We first identify a genomic region, RADres, showing the highest differentiation between susceptible and resistant inbred lines among 1611 informative restriction-site associated DNA (RAD) markers, and show that it significantly influences resistance in an independent set of 439 outbred snails. The additive effect of each RADres resistance allele is 2-fold, similar to that of the previously identified resistance gene sod1. The data fit a model in which both loci contribute independently and additively to resistance, such that the odds of infection in homozygotes for the resistance alleles at both loci (13% infected) is 16-fold lower than the odds of infection in snails without any resistance alleles (70% infected). Genome-wide linkage disequilibrium is high, with both sod1 and RADres residing on haplotype blocks >2 Mb, and with other markers in each block also showing significant effects on resistance; thus the causal genes within these blocks remain to be demonstrated. Other candidate loci had no effect on resistance, including the Guadeloupe Resistance Complex and three genes (aif, infPhox, and prx1) with immunological roles and expression patterns tied to resistance, which must therefore be trans-regulated.
Conclusions/significance: The loci RADres and sod1 both have strong effects on resistance to S. mansoni. Future approaches to control schistosomiasis may benefit from further efforts to characterize and harness this natural genetic variation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Resistance of Biomphalaria glabrata 13-16-R1 snails to Schistosoma mansoni PR1 is a function of haemocyte abundance and constitutive levels of specific transcripts in haemocytes.Int J Parasitol. 2014 May;44(6):343-53. doi: 10.1016/j.ijpara.2013.11.004. Epub 2014 Mar 28. Int J Parasitol. 2014. PMID: 24681237 Free PMC article.
-
Clusters of polymorphic transmembrane genes control resistance to schistosomes in snail vectors.Elife. 2020 Aug 26;9:e59395. doi: 10.7554/eLife.59395. Elife. 2020. PMID: 32845238 Free PMC article.
-
Epigenetic modulation, stress and plasticity in susceptibility of the snail host, Biomphalaria glabrata, to Schistosoma mansoni infection.Int J Parasitol. 2016 Jun;46(7):389-94. doi: 10.1016/j.ijpara.2016.03.003. Epub 2016 Apr 4. Int J Parasitol. 2016. PMID: 27056272 Review.
-
Identification and characterisation of functional expressed sequence tags-derived simple sequence repeat (eSSR) markers for genetic linkage mapping of Schistosoma mansoni juvenile resistance and susceptibility loci in Biomphalaria glabrata.Int J Parasitol. 2013 Jul;43(8):669-77. doi: 10.1016/j.ijpara.2013.03.007. Epub 2013 May 3. Int J Parasitol. 2013. PMID: 23643514 Free PMC article.
-
Molecular approaches in the study of Biomphalaria glabrata--Schistosoma mansoni interactions: linkage analysis and gene expression profiling.Parasitology. 2001;123 Suppl:S181-96. doi: 10.1017/s0031182001008174. Parasitology. 2001. PMID: 11769282 Review.
Cited by
-
A haplotype-like, chromosome-level assembled and annotated genome of Biomphalaria glabrata, an important intermediate host of schistosomiasis and the best studied model of schistosomiasis vector snails.PLoS Negl Trop Dis. 2024 Feb 29;18(2):e0011983. doi: 10.1371/journal.pntd.0011983. eCollection 2024 Feb. PLoS Negl Trop Dis. 2024. PMID: 38421953 Free PMC article.
-
Nuclear genome of Bulinus truncatus, an intermediate host of the carcinogenic human blood fluke Schistosoma haematobium.Nat Commun. 2022 Feb 21;13(1):977. doi: 10.1038/s41467-022-28634-9. Nat Commun. 2022. PMID: 35190553 Free PMC article.
-
Genetic Crosses and Linkage Mapping in Schistosome Parasites.Trends Parasitol. 2018 Nov;34(11):982-996. doi: 10.1016/j.pt.2018.08.001. Epub 2018 Aug 24. Trends Parasitol. 2018. PMID: 30150002 Free PMC article. Review.
-
A Targeted Capture Linkage Map Anchors the Genome of the Schistosomiasis Vector Snail, Biomphalaria glabrata.G3 (Bethesda). 2017 Jul 5;7(7):2353-2361. doi: 10.1534/g3.117.041319. G3 (Bethesda). 2017. PMID: 28526730 Free PMC article.
-
New Insights Into Biomphalysin Gene Family Diversification in the Vector Snail Biomphalaria glabrata.Front Immunol. 2021 Apr 1;12:635131. doi: 10.3389/fimmu.2021.635131. eCollection 2021. Front Immunol. 2021. PMID: 33868258 Free PMC article.
References
-
- Rottschaefer SM, Riehle MM, Coulibaly B, Sacko M, Niaré O, Morlais I, et al. Exceptional diversity, maintenance of polymorphism, and recent directional selection on the APL1 malaria resistance genes of Anopheles gambiae . PLoS Biol. 2011; 9: e1000600 10.1371/journal.pbio.1000600 - DOI - PMC - PubMed
-
- King CH, Sturrock RF, Kariuki HC, Hamburger J. Transmission control for schistosomiasis—why it matters now. Trends Parasitol. 2006; 22: 575–582. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

