Characterisation of a Novel Anti-CD52 Antibody with Improved Efficacy and Reduced Immunogenicity
- PMID: 26372145
- PMCID: PMC4570798
- DOI: 10.1371/journal.pone.0138123
Characterisation of a Novel Anti-CD52 Antibody with Improved Efficacy and Reduced Immunogenicity
Abstract
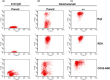
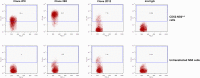
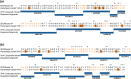
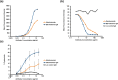
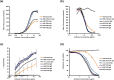
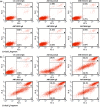
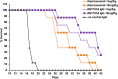
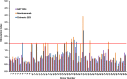
Anti-CD52 therapy has been shown to be effective in the treatment of a number of B cell malignancies, hematopoietic disorders and autoimmune diseases (including rheumatoid arthritis and multiple sclerosis); however the current standard of treatment, the humanized monoclonal antibody alemtuzumab, is associated with the development of anti-drug antibodies in a high proportion of patients. In order to address this problem, we have identified a novel murine anti-CD52 antibody which has been humanized using a process that avoids the inclusion within the variable domains of non-human germline MHC class II binding peptides and known CD4+ T cell epitopes, thus reducing its potential for immunogenicity in the clinic. The resultant humanized antibody, ANT1034, was shown to have superior binding to CD52 expressing cells than alemtuzumab and was more effective at directing both antibody dependent and complement dependent cell cytotoxicity. Furthermore, when in the presence of a cross-linking antibody, ANT1034 was more effective at directly inducing apoptosis than alemtuzumab. ANT1034 also showed superior activity in a SCID mouse/human CD52 tumour xenograft model where a single 1 mg/Kg dose of ANT1034 led to increased mouse survival compared to a 10 mg/Kg dose of alemtuzumab. Finally, ANT1034 was compared to alemtuzumab in in vitro T cell assays in order to evaluate its potential to stimulate proliferation of T cells in peripheral blood mononuclear cells derived from a panel of human donors: whereas alemtuzumab stimulated proliferation in a high proportion of the donor cohort, ANT1034 did not stimulate proliferation in any of the donors. Therefore we have developed a candidate therapeutic humanized antibody, ANT1034, that may have the potential to be more efficacious and less immunogenic than the current standard anti-CD52 therapy.
Conflict of interest statement
Figures

Similar articles
-
Depletion of CD52-positive cells inhibits the development of central nervous system autoimmune disease, but deletes an immune-tolerance promoting CD8 T-cell population. Implications for secondary autoimmunity of alemtuzumab in multiple sclerosis.Immunology. 2017 Apr;150(4):444-455. doi: 10.1111/imm.12696. Epub 2017 Jan 3. Immunology. 2017. PMID: 27925187 Free PMC article.
-
A novel Raji-Burkitt's lymphoma model for preclinical and mechanistic evaluation of CD52-targeted immunotherapeutic agents.Clin Cancer Res. 2008 Jan 15;14(2):569-78. doi: 10.1158/1078-0432.CCR-07-1006. Clin Cancer Res. 2008. PMID: 18223233
-
In vitro and in vivo evaluation of direct rhenium-188-labeled anti-CD52 monoclonal antibody alemtuzumab for radioimmunotherapy of B-cell chronic lymphocytic leukemia.Nucl Med Biol. 2008 Jul;35(5):599-604. doi: 10.1016/j.nucmedbio.2008.03.001. Nucl Med Biol. 2008. PMID: 18589304
-
Alemtuzumab for the prevention and treatment of graft-versus-host disease.Int J Hematol. 2011 May;93(5):586-593. doi: 10.1007/s12185-011-0802-2. Epub 2011 Mar 3. Int J Hematol. 2011. PMID: 21369856 Review.
-
Alemtuzumab: evidence for its potential in relapsing-remitting multiple sclerosis.Drug Des Devel Ther. 2013;7:131-8. doi: 10.2147/DDDT.S32687. Epub 2013 Mar 6. Drug Des Devel Ther. 2013. PMID: 23494602 Free PMC article. Review.
Cited by
-
CD52-targeted depletion by Alemtuzumab ameliorates allergic airway hyperreactivity and lung inflammation.Mucosal Immunol. 2021 Jul;14(4):899-911. doi: 10.1038/s41385-021-00388-5. Epub 2021 Mar 17. Mucosal Immunol. 2021. PMID: 33731828 Free PMC article.
-
The INNs and outs of antibody nonproprietary names.MAbs. 2016;8(1):1-9. doi: 10.1080/19420862.2015.1114320. MAbs. 2016. PMID: 26716992 Free PMC article.
-
Mechanisms of Action and Limitations of Monoclonal Antibodies and Single Chain Fragment Variable (scFv) in the Treatment of Cancer.Biomedicines. 2023 Jun 1;11(6):1610. doi: 10.3390/biomedicines11061610. Biomedicines. 2023. PMID: 37371712 Free PMC article. Review.
-
Specificity of the T Cell Response to Protein Biopharmaceuticals.Front Immunol. 2020 Jul 22;11:1550. doi: 10.3389/fimmu.2020.01550. eCollection 2020. Front Immunol. 2020. PMID: 32793213 Free PMC article. Review.
-
The Irony of Humanization: Alemtuzumab, the First, But One of the Most Immunogenic, Humanized Monoclonal Antibodies.Front Immunol. 2020 Feb 14;11:124. doi: 10.3389/fimmu.2020.00124. eCollection 2020. Front Immunol. 2020. PMID: 32117274 Free PMC article.
References
-
- Hale G. The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy. Elsevier; 2001. January;3(3):137–43. - PubMed
-
- Watanabe T, Masuyama J, Sohma Y, Inazawa H, Horie K, Kojima K, et al. CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. Clin Immunol. 2006. September - PubMed
-
- Rowan WC, Hale G, Tite JP, Brett SJ. Cross-linking of the CAMPATH-1 antigen (CD52) triggers activation of normal human T lymphocytes. Int Immunol. 1995. January - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

