dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis
- PMID: 26372309
- PMCID: PMC4579786
- DOI: 10.1038/ncomms9244
dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis
Abstract
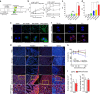
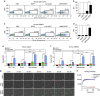
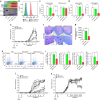
Central nervous system (CNS)-infiltrating effector T cells play critical roles in the development and progression of multiple sclerosis (MS). However, current drugs for MS are very limited due to the difficulty of delivering drugs into the CNS. Here we identify a cell-permeable peptide, dNP2, which efficiently delivers proteins into mouse and human T cells, as well as various tissues. Moreover, it enters the brain tissue and resident cells through blood vessels by penetrating the tightly organized blood-brain barrier. The dNP2-conjugated cytoplasmic domain of cytotoxic T-lymphocyte antigen 4 (dNP2-ctCTLA-4) negatively regulates activated T cells and shows inhibitory effects on experimental autoimmune encephalomyelitis in both preventive and therapeutic mouse models, resulting in the reduction of demyelination and CNS-infiltrating T helper 1 and T helper 17 cells. Thus, this study demonstrates that dNP2 is a blood-brain barrier-permeable peptide and dNP2-ctCTLA-4 could be an effective agent for treating CNS inflammatory diseases such as MS.
Conflict of interest statement
S. L., W.-J.K., Y.-H.K., and J.-M.C. are inventors on a patent application describing the use of dNP2 as drug delivery peptide and therapeutic use of dNP2-ctCTLA-4 in multiple sclerosis. The remaining authors declare no competing financial interests.
Figures


Similar articles
-
LRR domain of NLRX1 protein delivery by dNP2 inhibits T cell functions and alleviates autoimmune encephalomyelitis.Theranostics. 2020 Feb 10;10(7):3138-3150. doi: 10.7150/thno.43441. eCollection 2020. Theranostics. 2020. PMID: 32194859 Free PMC article.
-
In Vivo Induction of Regulatory T Cells Via CTLA-4 Signaling Peptide to Control Autoimmune Encephalomyelitis and Prevent Disease Relapse.Adv Sci (Weinh). 2021 May 5;8(14):2004973. doi: 10.1002/advs.202004973. eCollection 2021 Jul. Adv Sci (Weinh). 2021. PMID: 34306974 Free PMC article.
-
Regulation of human T cell responses by dNP2-ctCTLA-4 inhibits human skin and microvessel graft rejection.Biomaterials. 2018 Nov;183:128-138. doi: 10.1016/j.biomaterials.2018.08.049. Epub 2018 Aug 21. Biomaterials. 2018. PMID: 30165256 Free PMC article.
-
Role of Mast Cells in the Pathogenesis of Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis.Clin Rev Allergy Immunol. 2017 Jun;52(3):436-445. doi: 10.1007/s12016-016-8595-y. Clin Rev Allergy Immunol. 2017. PMID: 28025778 Review.
-
Th17 Cells in MS and Experimental Autoimmune Encephalomyelitis.Int MS J. 2009 Apr;16(1):12-8. Int MS J. 2009. PMID: 19413921 Review.
Cited by
-
Alleviating psoriatic skin inflammation through augmentation of Treg cells via CTLA-4 signaling peptide.Front Immunol. 2023 Sep 25;14:1233514. doi: 10.3389/fimmu.2023.1233514. eCollection 2023. Front Immunol. 2023. PMID: 37818377 Free PMC article.
-
The Role of Cytotoxic T-Lymphocyte Antigen 4 in the Pathogenesis of Multiple Sclerosis.Genes (Basel). 2022 Jul 24;13(8):1319. doi: 10.3390/genes13081319. Genes (Basel). 2022. PMID: 35893056 Free PMC article. Review.
-
Efficient intracellular delivery of proteins by a multifunctional chimaeric peptide in vitro and in vivo.Nat Commun. 2021 Aug 26;12(1):5131. doi: 10.1038/s41467-021-25448-z. Nat Commun. 2021. PMID: 34446736 Free PMC article.
-
Effect of Aminated Mesoporous Bioactive Glass Nanoparticles on the Differentiation of Dental Pulp Stem Cells.PLoS One. 2016 Mar 14;11(3):e0150727. doi: 10.1371/journal.pone.0150727. eCollection 2016. PLoS One. 2016. PMID: 26974668 Free PMC article.
-
Airborne fine particles drive H1N1 viruses deep into the lower respiratory tract and distant organs.Sci Adv. 2023 Jun 9;9(23):eadf2165. doi: 10.1126/sciadv.adf2165. Epub 2023 Jun 9. Sci Adv. 2023. PMID: 37294770 Free PMC article.
References
-
- Trapp B. D., Ransohoff R. & Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr. Opin. Neurol. 12, 295–302 (1999). - PubMed
-
- Wingerchuk D. M. & Carter J. L. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin. Proc. 89, 225–240 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

