Covered metallic stents with an anti-migration design vs. uncovered stents for the palliation of malignant gastric outlet obstruction: a multicenter, randomized trial
- PMID: 26372507
- PMCID: PMC4697131
- DOI: 10.1038/ajg.2015.286
Covered metallic stents with an anti-migration design vs. uncovered stents for the palliation of malignant gastric outlet obstruction: a multicenter, randomized trial
Abstract
Objectives: Previous studies reported comparable stent patency between covered self-expandable metallic stents (SEMS) and uncovered SEMS (UCS) for palliation of malignant gastric outlet obstruction (GOO). The aim of this study was to evaluate the efficacy and safety of the newly developed WAVE-covered SEMS (WCS), which has an anti-migration design, compared with UCS in gastric cancer patients with symptomatic GOO.
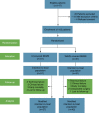
Methods: A total of 102 inoperable gastric cancer patients with symptomatic GOO were prospectively enrolled from five referral centers and randomized to undergo UCS or WCS placement. Stent patency and recurrence of obstructive symptoms were assessed at 8 weeks and 16 weeks after stent placement.
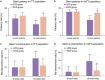
Results: At the 8-week follow-up, both stent patency rates (72.5% vs. 62.7%) and re-intervention rates (19.6% vs. 19.6%) were comparable between the WCS and the UCS groups. Both stent stenosis (2.4% vs. 8.1%) and migration rates (9.5% vs. 5.4%) were comparable between WCS and UCS groups. At the 16-week follow-up, however, the WCS group had a significantly higher stent patency rate than the UCS group (68.6% vs. 41.2%). Re-intervention rates in the WCS and UCS groups were 23.5% and 39.2%, respectively. Compared with the UCS group, the WCS group had a significantly lower stent restenosis rate (7.1% vs. 37.8%) and a comparable migration rate (9.5% vs. 5.4%). Overall stent patency was significantly longer in the WCS group than in the UCS group. No stent-associated significant adverse events occurred in either the WCS or UCS groups. In the multivariate analysis, WCS placement and chemotherapy were identified as independent predictors of 16-week stent patency.
Conclusions: WCS group showed comparable migration rate and significantly more durable long-term stent patency compared with UCS group for the palliation of GOO in patients with inoperable gastric cancer.
Figures


References
-
- 1Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 2001;344:1681–1687. - PubMed
-
- 2Dormann A, Meisner S, Verin N et al. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy 2004;36:543–550. - PubMed
-
- 3Yim HB, Jacobson BC, Saltzman JR et al. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc 2001;53:329–332. - PubMed
-
- 4Brimhall B, Adler DG. Enteral stents for malignant gastric outlet obstruction. Gastrointest Endosc Clin N Am 2011;21:389–403. - PubMed
-
- 5Jeurnink SM, Steyerberg EW, van Hooft JE et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc 2010;71:490–499. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

