Differential expression of HDAC and HAT genes in atrophying skeletal muscle
- PMID: 26372908
- PMCID: PMC4715560
- DOI: 10.1002/mus.24912
Differential expression of HDAC and HAT genes in atrophying skeletal muscle
Abstract
Introduction: Histone deacetylase (HDAC) proteins, which counter the activity of histone acetyltransferases (HATs), are necessary for normal muscle atrophy in response to several pathophysiological conditions. Despite this, it remains unknown whether a common or unique transcriptional profile of HDAC and HAT genes exist during the progression of muscle atrophy.
Methods: Muscles were harvested from cast immobilized, denervated, or nutrient deprived animals for quantitative reverse transcriptase-polymerase chain reaction analysis of HDAC and HAT gene expression.
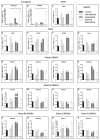
Results: The mRNA levels of Hdac2, Hdac4, Hdac6, Sirt1, p300, Cbp, and Pcaf increased, and Hdac7 decreased in skeletal muscle in each experimental model of muscle atrophy. Hdac1 and Hdac3 were increased only in cast immobilized and denervated muscles.
Conclusions: While specific HDACs and HATs are increased in multiple models of muscle atrophy, increased expression of class I HDACs was unique to muscle disuse, reinforcing that specific HDAC inhibitors may be more effective than pan-HDAC inhibitors at countering muscle atrophy.
Keywords: denervation; histone acetyltransferase; histone deacetylase; muscle disuse; muscle wasting.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
No conflict of interests
Figures
References
-
- Demos-Davies KM, Ferguson BS, Cavasin MA, Mahaffey JH, Williams SM, Spiltoir JI, Schuetze KB, Horn TR, Chen B, Ferrara C, Scellini B, Piroddi N, Tesi C, Poggesi C, Jeong MY, McKinsey TA. HDAC6 contributes to pathological responses of heart and skeletal muscle to chronic angiotensin-II signaling. American journal of physiology Heart and circulatory physiology. 2014;307(2):H252–258. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

