Structural insight into selectivity and resistance profiles of ROS1 tyrosine kinase inhibitors
- PMID: 26372962
- PMCID: PMC4593101
- DOI: 10.1073/pnas.1515281112
Structural insight into selectivity and resistance profiles of ROS1 tyrosine kinase inhibitors
Abstract
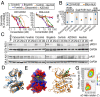
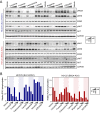
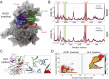
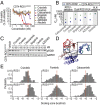
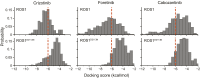
Oncogenic ROS1 fusion proteins are molecular drivers in multiple malignancies, including a subset of non-small cell lung cancer (NSCLC). The phylogenetic proximity of the ROS1 and anaplastic lymphoma kinase (ALK) catalytic domains led to the clinical repurposing of the Food and Drug Administration (FDA)-approved ALK inhibitor crizotinib as a ROS1 inhibitor. Despite the antitumor activity of crizotinib observed in both ROS1- and ALK-rearranged NSCLC patients, resistance due to acquisition of ROS1 or ALK kinase domain mutations has been observed clinically, spurring the development of second-generation inhibitors. Here, we profile the sensitivity and selectivity of seven ROS1 and/or ALK inhibitors at various levels of clinical development. In contrast to crizotinib's dual ROS1/ALK activity, cabozantinib (XL-184) and its structural analog foretinib (XL-880) demonstrate a striking selectivity for ROS1 over ALK. Molecular dynamics simulation studies reveal structural features that distinguish the ROS1 and ALK kinase domains and contribute to differences in binding site and kinase selectivity of the inhibitors tested. Cell-based resistance profiling studies demonstrate that the ROS1-selective inhibitors retain efficacy against the recently reported CD74-ROS1(G2032R) mutant whereas the dual ROS1/ALK inhibitors are ineffective. Taken together, inhibitor profiling and stringent characterization of the structure-function differences between the ROS1 and ALK kinase domains will facilitate future rational drug design for ROS1- and ALK-driven NSCLC and other malignancies.
Keywords: ALK; ROS1; inhibitor; kinase; structural modelling.
Conflict of interest statement
Conflict of interest statement: The Oregon Health & Science University has clinical trial contracts with Novartis and Bristol-Myers Squibb (BMS) to pay for patient costs, nurse and data manager salaries, and institutional overhead. B.J.D. does not derive salary, nor does his laboratory receive funds, from these contracts. M.W.D. served on advisory boards and as a consultant for BMS, ARIAD, and Novartis.
Figures




Comment in
-
Clinical activity of ceritinib in ROS1-rearranged non-small cell lung cancer: Bench to bedside report.Proc Natl Acad Sci U S A. 2016 Mar 15;113(11):E1419-20. doi: 10.1073/pnas.1522052113. Epub 2016 Feb 25. Proc Natl Acad Sci U S A. 2016. PMID: 26917690 Free PMC article. No abstract available.
References
-
- Druker BJ. Perspectives on the development of imatinib and the future of cancer research. Nat Med. 2009;15(10):1149–1152. - PubMed
-
- Druker BJ, et al. IRIS Investigators Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. - PubMed
-
- Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. - PubMed
-
- Grande E, Bolós MV, Arriola E. Targeting oncogenic ALK: A promising strategy for cancer treatment. Mol Cancer Ther. 2011;10(4):569–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

