Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions
- PMID: 26373371
- PMCID: PMC5308417
- DOI: 10.1038/nrmicro3525
Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions
Abstract
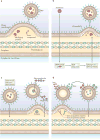
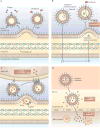
Outer-membrane vesicles (OMVs) are spherical buds of the outer membrane filled with periplasmic content and are commonly produced by Gram-negative bacteria. The production of OMVs allows bacteria to interact with their environment, and OMVs have been found to mediate diverse functions, including promoting pathogenesis, enabling bacterial survival during stress conditions and regulating microbial interactions within bacterial communities. Additionally, because of this functional versatility, researchers have begun to explore OMVs as a platform for bioengineering applications. In this Review, we discuss recent advances in the study of OMVs, focusing on new insights into the mechanisms of biogenesis and the functions of these vesicles.
Conflict of interest statement
statement The authors declare no competing interests.
Figures


References
-
- Zhou L, Srisatjaluk R, Justus DE, Doyle RJ. On the origin of membrane vesicles in Gram-negative bacteria. FEMS Microbiol Lett. 1998;163:223–228. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

